
news
新闻中心
Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer
Recommendations for HER2 Testing in Breast Cancer: ASCO/CAP Guideline Update
Intervention
• Recommendations for HER2 testing in breast cancer
Target Audience
• Medical oncologists, pathologists, and surgeons
Key Recommendations for Oncologists
• Must request HER2 testing on every primary invasive breast cancer (and on metastatic site, if stage IV and if specimen available) from a patient with breast cancer to guide decision to pursue HER2-targeted therapy. This should be especially considered for a patient who previously tested HER2 negative in a primary tumor and presents with disease recurrence with clinical behavior suggestive of HER2-positive or triple-negative disease.
• Should recommend HER2-targeted therapy if HER2 test result is positive, if there is no apparent histopathologic discordance with HER2 testing (Tables 1 and 2), and if clinically appropriate. If the pathologist or oncologist observes an apparent histopathologic discordance after HER2 testing, the need for additional HER2 testing should be discussed.
• Must delay decision to recommend HER2-targeted therapy if initial HER2 test result is equivocal. Reflex testing should be performed on the same specimen using the alternative test if initial HER2 test result is equivocal or on an alternative specimen (Tables 1 and 2).
• Must not recommend HER2-targeted therapy if HER2 test result is negative and if there is no apparent histopathologic discordance with HER2 testing (Tables 1 and 2). If the pathologist or oncologist observes an apparent histopathologic discordance after HER2 testing, the need for additional HER2 testing should be discussed.
• Should delay decision to recommend HER2-targeted therapy if HER2 status cannot be confirmed as positive or negative after separate HER2 tests (HER2 test result or results equivocal). The oncologist should confer with the pathologist regarding the need for additional HER2 testing on the same or another tumor specimen.
• If the HER2 test result is ultimately deemed to be equivocal, even after reflex testing with an alternative assay (ie, if neither test is unequivocally positive), the oncologist may consider HER2-targeted therapy. The oncologist should also consider the feasibility of testing another tumor specimen to attempt to definitely establish the tumor HER2 status and guide therapeutic decisions. A clinical decision to ultimately consider HER2-targeted therapy in such cases should be individualized on the basis of patient status (comorbidities, prognosis, and so on) and patient preferences after discussing available clinical evidence.
Key Recommendations for Pathologists
• Must ensure that at least one tumor sample from all patients with breast cancer (early-stage or metastatic disease) is tested for either HER2 protein expression (IHC assay) or HER2 gene expression (ISH assay) using a validated HER2 test.
• In the United States, the ASCO/CAP Guideline Update Committee preferentially recommends the use of an assay that has received FDA approval, although a CLIA-certified laboratory may choose instead to use a laboratory-developed test (LDT). In this case, the analytic performance of the LDT must beprospectively validated in the same clinical laboratory that will perform it, and the test must have documented analytic validity (CAP guidance document).
Bright-field ISH assays must be initially validated by comparing them with an FDA-approved FISH assay.
• Must report a HER2 test result as positive if: (a) IHC 3+ positive or (b) ISH positive using either a single probe ISH or dual-probe ISH (Table 1; Figs 1 to 3). This assumes that there is no apparent histopathologic discordance observed by the pathologist (Table 2).
• Must report a HER2 test result as equivocal and order reflex test on the same specimen (unless the pathologist has concerns about the specimen) using the alternative test if: (a) IHC 2+ equivocal or (b) ISH equivocal using single-probe ISH or dual-probe ISH (Table 1; Figs 1 to 3). This assumes that there is no apparent histopathologic discordance observed by the pathologist (Table 2). Note that there are some rare breast cancers (eg, gland-forming tumors,micropapillary carcinomas) that show IHC 1+ staining that is intense but incomplete (basolateral or U shaped) and that are found to be HER2 amplified.The pathologist should consider also reporting these specimens equivocal andrequest reflex testing using the alternative test.
• Must report a HER2 test result as negative if a single test (or all tests)performed on a tumor specimen show: (a) IHC 1 + negative or IHC 0 negative or (b) ISH negative using single-probe ISH or dual-probe ISH (Table 1; Figs 1 to 3). This assumes that there is no apparent histopathologic discordance observed by the pathologist (Table 2).
• Must report a HER2 test result as indeterminate if technical issues prevent one or both tests (IHC and ISH) performed on a tumor specimen from being reported as positive, negative, or equivocal. This may occur if specimen handling was inadequate, if artifacts (crush or edge artifacts) make interpretation difficult, or if the analytic testing failed. Another specimen should be requested for testing, if possible, and a comment should be included in the pathology report documenting intended action.
Arch Pathol Lab Med. Author manuscript; available in PMC 2014 July 08.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript
• Must ensure that interpretation and reporting guidelines for HER2 testing are followed (Table 1; Data Supplements 7, 8, 9, and 10).
• Should interpret bright-field ISH on the basis of a comparison between patterns in normal breast and tumor cells, because artifactual patterns may be seen that are difficult to interpret. If tumor cell pattern is neither normal nor clearly amplified, test should be submitted for expert opinion.
• Should ensure that any specimen used for HER2 testing (cytologic specimens, needle biopsies, or resection specimens) begins the fixation process quickly (time to fixative within 1 hour) and is fixed in 10% neutral buffered formalin for 6 to 72 hours and that routine processing, as well as staining or probing, is performed according to standardized analytically validated protocols.
• Should ensure that the laboratory conforms to standards set for CAP accreditation or an equivalent accreditation authority, including initial test validation, ongoing internal quality assurance, ongoing external proficiency testing, and routine periodic performance monitoring.
• If an apparent histopathologic discordance is observed in any HER2 testing situation (Table 2), the pathologist should consider ordering additional HER2 testing and conferring with the oncologist, and should document the decision- making process and results in the pathology report. As part of the HER2 testing process, the pathologist may pursue additional HER2 testing without conferring with the oncologist.
• Although categories of HER2 status by IHC or ISH can be created that are not covered by these definitions, in practice they are uncommon and if encountered should be considered IHC equivocal or ISH equivocal.
• Systematic review and analysis of the medical literature were conducted by the 2013 Update Committee.
Additional Information
• The revised recommendations and a brief summary of the literature and analysis are provided in this article. Data Supplements including clinical tools and resources can be found at www.asco.org/guidelines/her2 and at http:// www.cap.org. Patient information is available at http://www.cancer.net. ASCO and CAP believe that cancer clinical trials are vital to inform medical decisions and improve cancer care, and that all patients should have the opportunity to participate.
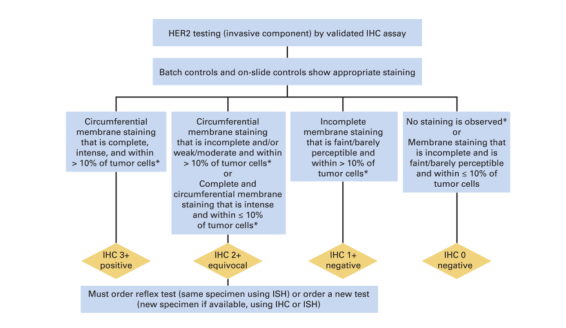
Algorithm for evaluation of human epidermal growth factor receptor 2 (HER2) protein expression by immunohistochemistry (IHC) assay of the invasive component of a breast cancer specimen. Although categories of HER2 status by IHC can be created that are not covered by these definitions, in practice they are rare and if encountered should be considered IHC 2+ equivocal. ISH, in situ hybridization. NOTE: the final reported results assume that there is no apparent histopathologic discordance observed by the pathologist. (*) Readily appreciated using a low-power objective and observed within a homogeneous and contiguous invasive cell population.
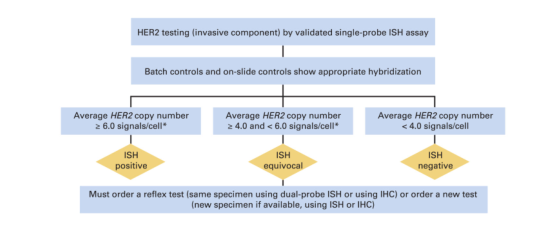
Algorithm for evaluation of human epidermal growth factor receptor 2 (HER2) gene amplification by in situ hybridization (ISH) assay of the invasive component of a breast cancer specimen using a single-signal (HER2 gene) assay (single-probe ISH). Amplification in a single-probe ISH assay is defined by examining the average HER2 copy number. If there is a second contiguous population of cells with increased HER2 signals per cell, and this cell population consists of more than 10% of tumor cells on the slide (defined by image analysis or visual estimation of the ISH or immunohistochemistry [IHC] slide), a separate counting of at least 20 nonoverlapping cells must also be performed within this cell population and also reported. Although categories of HER2 status by ISH can be created that are not covered by these definitions, in practice they are rare and if encountered should be considered ISH equivocal (see Data Supplement 2E). NOTE: the final reported results assume that there is no apparent histopathologic discordance observed by the pathologist. (*)Observed in a homogeneous and contiguous population.
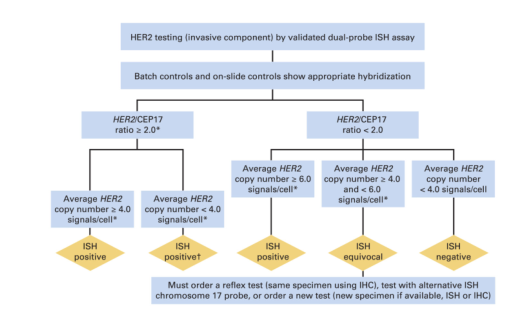
Algorithm for evaluation of human epidermal growth factor receptor 2 (HER2) gene amplification by in situ hybridization (ISH) assay of the invasive component of a breast cancer specimen using a dual-signal (HER2 gene) assay (dual-probe ISH). Amplification in a dual-probe ISH assay is defined by examining first the HER2/CEP17 ratio followed by the average HER2 copy number (see Data Supplement 2E for more details). If there is a second contiguous population of cells with increased HER2 signals per cell, and this cell population consists of more than 10% of tumor cells on the slide (defined by image analysis or visual estimation of the ISH or immunohistochemistry [IHC] slide), a separate counting of at least 20 nonoverlapping cells must also be performed within this cell population and also reported. Although categories of HER2 status by ISH can be created that are not covered by these definitions, in practice they are rare and if encountered should be considered ISH equivocal (see Data Supplement 2E). NOTE. The final reported results assume that there is no apparent histopathologic discordance observed by the pathologist. (*) Observed in a homogeneous and contiguous population. (†) See Data Supplement 2E for more information on these rare scenarios.
Wolff et al. Page 29
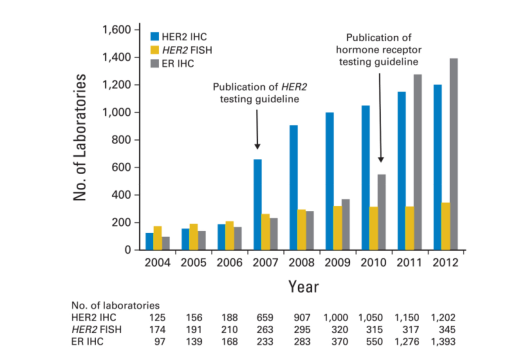
Figure 4.
Number of laboratories participating in predictive marker proficiency testing for human epidermal growth factor receptor 2 (HER2) by immunohistochemistry (IHC), HER2 by fluorescent in situ hybridization (FISH), and estrogen receptor (ER) by IHC through the College of American Pathologists (CAP) Laboratory Improvement Program. Arrows indicate the years during which the HER2 and hormone receptor testing guidelines were published by the American Society of Clinical Oncology (ASCO)/CAP. The numbers of participating laboratories are shown both graphically and in tabular form. After the publication of the 2007 ASCO/CAP HER2 and the 2010 ASCO/CAP ER/progesterone receptor testing guidelines, there was a significant increase in the number of laboratories in the United States and elsewhere participating in CAP proficiency testing surveys in breast cancer (http://www.cap.org/apps/cap.portal_nfpb=true&_pageLabel=accreditation;last check-ed June 14, 2013). CAP has a core goal to improve the quality of pathology and laboratory services through education and standard setting in order to enhance patient safety, and help laboratories meet or exceed regulatory requirements set by the Centers for Medicare and Medicaid Services, the Joint Commission, and many states in the United States.