news
新闻中心
Paediatric non-Hodgkin lymphoma - perspectives in translational
Bruce Shiramizu 1 , Lara Mussolin 2,3 , Wilhelm Woessmann 4,5 , and Wolfram Klapper 6
1 John A. Burns School of Medicine, Department of Pediatrics, University of Hawaii, Honolulu,USA
2 University of Padova, Department of Woman and Child Health, via Giustiniani 3, 35120, Padova,Italy
3 IRP-Istituto di Ricerca Pediatrica-Cittàdella Speranza, Corso StatiUniti 4, 35121, Padova, Italy
4 Department of Paediatric Haematology and Oncology, Justus-Liebig-University, Giessen,Germany
5 Non-Hodgkin Lymphoma-Berlin-Frankfurt-Münster Study Centre, Department of Paediatric Haematology and Oncology, Justus-Liebig University, Giessen, Germany
6 Department of Pathology, Haematopathology Section, University-Hospital Schleswig-Holstein,University of Kiel, Germany
Summary
Exciting advances have been achieved for infants, children and adolescents diagnosed with, and treated for, non-Hodgkin lymphoma (NHL). In spite of these successes, new frontiers are being paved to improve the prognosis for those who relapse or have resistant disease. This review summarizes some of the novel approaches and ideas in NHL monitoring, diagnosis and treatment as discussed at the 5th International Symposium on Childhood, Adolescent and Young Adult Non-Hodgkin Lymphoma on October 22 nd to 24 th 2015 in Varese, Italy.
Keywords
non-Hodgkin lymphoma; lymphoma; childhood lymphoma
Introduction
The prognosis for infants, children and adolescents diagnosed with non-Hodgkin lymphoma (NHL) has significantly improved over the last 2 decades. However 10–30% of these patients will relapse. While there are treatment options available for those patients whose disease recurs, the outcomes remain dismal. Thus therapy strategies focusing on recurrent disease present further challenges. Future successes in the treatment, prognosis and understanding paediatric NHL will rely, amongst other factors, on improvements in monitoring for minimal disseminated disease (MDD) and/or minimal residual disease (MRD) and advances in molecular diagnosis which could lead to more molecular and/or immunotargeted therapy. This review will highlight advances in paediatric and adolescent NHL with an emphasis on future strategies and research directions.
Molecular monitoring of disease activity and tumour burden
In children with lymphoblastic leukaemia, examination of the bone marrow (BM) at diagnosis and during therapy is an integral part of their clinical management. The prognostic impact of treatment response overrides other clinical risk factors. MRD is defined as the residual disease detectable in patient’s BM or peripheral blood below the detection limit of the available conventional methods. In leukaemia patients, MRD monitoring is currently considered the most reliable strategy to evaluate early treatment response and to refine stratification accordingly (Bhojwani and Pui 2013, Cave, et al 1998, Pui, et al 2012, Tallen,et al 2010).
Several techniques have been developed over the past 15 years to complement morphology in assessing response to treatment, especially MRD, including immunological, molecular and fluorescent in situ hybridization (FISH) assays (Cazzaniga, et al 2002, Neale, et al 2003, Szczepanski, et al 2010). However, most of these techniques have limited sensitivity or applicability. Reliable techniques to detect MRD should discriminate between malignant and normal cells, the tumour-specific markers should be stable and they should have a sensitivity of at least 10 −4 (1 malignant cell within 10 4 normal cells). The established methods for MRD detection in leukaemia include flow cytometric analysis of aberrant immunophenotypes, polymerase chain reaction (PCR) amplification of fusion transcripts or antigen receptor genes.
MRD could not be assessed in NHL for a long time because markers often need to be developed from the primary tumour. However, fresh material is often not available and MDD-detection is a prerequisite for MRD-measurement in NHL. During the last few years, different studies have demonstrated that MDD and MRD in paediatric NHL could be a powerful tool for stratifying patients in different prognostic groups and monitoring the treatment response (Agsalda, et al 2009, Coustan-Smith, et al 2009, Damm-Welk, et al 2007, Damm-Welk, et al 2014, Mussolin, et al 2013, Mussolin, et al 2011).
The methodologies, timing of MRD assessment and sensitivity required for informative MDD/MRD studies depend mainly on the NHL subtype and clinical question, and also on the expertise and the facilities available. Standardization and inter-laboratory quality control of these methods are absolutely needed to use biological stratification criteria in international studies. In most NHL protocols to date, the treatment plan was not adapted to biological risk factors but rather stage and tumour burden. Anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL) is a good example for which biological criteria are becoming an integral part of the management of patients. Until recently, BM involvement was considered a rare event in ALCL, due to the subtle nature of the BM involvement and the difficulty of its detection by routine morphological examination. Ninety per cent of ALK-positive ALCL carry the t(2;5)(p23;q35) chromosomal translocation, which gives rise to the fusion gene NPM1-ALK . The fusion gene transcript can be readily and sensitively detected by PCR. An international study performed in a large cohort of uniformly treated children with ALK-positive ALCL demonstrated MDD- positivity detected by PCR at diagnosis in BM or peripheral blood, in 59% of the patients. MDD-positivity conferred a relapse risk of about 50% (Mussolin et al , 2013). Only MDD positivity together with low antibody titre anti-ALK and histology other than common type were predictive of higher risk of failure (hazard ratio 4.9 and 2.7, respectively) in multivariate analysis (Mussolin et al , 2013). Furthermore, the persistence of MRD early during treatment identified patients with a very high risk of relapse (80%) (Damm-Welk, et al 2014). These studies led to the decision to use MDD for risk stratification in the next ALCL protocol of the European Intergroup for Childhood non-Hodgkin Lymphoma (EICNHL) with risk -adapted therapy. MDD is measured in five national laboratories according to standard operating procedures (SOPs) and routine quality control for NPM1-ALK reverse transcription (RT)-PCR measurement has been successfully established among these reference laboratories. This effort is fundamental to ensure reproducible results within multicentre international studies. Newly developed technologies, such as next generation sequencing and digital PCR, which present benefits for MRD/MDD detection still need to demonstrate their technical advantage compared to the established assays before a switch in technology seems reasonable.
The latest data from gene expression profiling, single nucleotide polymorphism arrays and, more recently, next generation sequencing, have expanded the full repertoire of genetic lesions in childhood NHL (Basso, et al 2011, Bonn, et al 2015, Salaverria, et al 2008). T-lymphoblastic lymphoma (T-LBL) is the leading disease entity that highlights this issue. Although many of the genomic studies have increased our knowledge of the pathogenesis of T-LBL, it is not known yet whether and how new specific genotypes will affect the clinical management of patients with T-LBL. Recent retrospective molecular studies in T-LBL have shown an association of loss of heterozygosity at chromosome 6q (LOH6q), biallelic T-cell receptor-γ deletions and FBXW7 and NOTCH1 mutations with outcome (Bonn, et al 2013, Callens, et al 2012). However, the very first biological prognostic parameter on T-LBL, reported by Coustan-Smith et al (2009) in 99 paediatric T-LBL patients, was MDD at diagnosis assessed by flow cytometry. Using a cut-off level of 1%, the 2-year event-free survival (EFS) was 68% for patients with higher levels of disease dissemination versus 91% for those with lower levels (Coustan-Smith, et al 2009). The prognostic role of MDD for LBL was recently confirmed in a large Italian cohort of pre-B and T-LBL cases (Mussolin,et al 2015).
It is likely that genomics will be translated into a better risk stratification that will allow for tailored therapies in the near future. However, several factors need to be considered before introducing a novel genetic discovery as stratification parameter. These include the treatment context, independent verification in other cohorts and/or prospective clinical trials, and independent prognostic values in multivariate analysis with MDD/MRD.
The St. Jude NHL staging classification for paediatric NHL was developed more than 35 years ago (Murphy 1980). An international multidisciplinary expert panel convened in Frankfurt, Germany, at the Third International Childhood, Adolescent and Young Adult NHL Symposium in 2009 to develop a revised international paediatric NHL staging system (IPNHLSS), addressing limitations of the current paediatric NHL staging system and creating a revised classification. The authors recognized that there is evidence that specific disease characteristics, including MDD, may have relevant influence on outcome and they defined subcategories that recognizes novel technologies for minimal disease quantification in BM and cerebral spinal fluid for future consideration (Rosolen, et al 2015). It is important to point out that this new NHL staging allows for data collection but does not change the stage or stratification based on the MDD finding. However it provides for opportunities for future analyses to be considered using the staging data, such as whether MDD might be useful for stratification in other subtypes.
Overall, risk stratification in future NHL clinical trials based on newly identified biological risk categories could provide future tailored treatment strategies to improve survival for high-risk patients while decreasing toxicity for low-risk patients. This can be accomplished through novel molecular diagnostics and characterizing lymphomas in children and adolescents.
Molecular diagnosis and subtyping of lymphomas in children
The technology to generate molecular data of tumour specimen has developed rapidly in recent decades. On one hand, these advances increased the complexity of data generated by using high-throughput technology, such as gene expression arrays and next-generation sequencing. On the other hand, broader applicability of molecular analyses is achieved through expanding access to research data. Furthermore, development of assays that allowed use of small amounts of biomaterial, such as cytological preparations as well as biomaterials of limited quantities from formalin-fixed and paraffin embedded (FFPE) tissues, has increased the spectrum of applicability. This development moved molecular profiling from a base research tool to clinical application and raised excitement that molecular analysis might achieve two goals: (i) improvement in diagnosis; and (ii) subdivision of entities into clinically relevant subgroups. However, insights into disease characteristics that can be translated into clinical application differ between adult and paediatric lymphomas. This difference is highlighted when differentiating mature aggressive B-cell lymphoma, such as Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL). Molecular diagnosis and subtyping appear more relevant in adults compared to children. In contrast, in T-LBL, molecular-genetic subtyping has almost exclusively been driven forward in paediatric lymphoma.
Molecular diagnosis of mature aggressive B-cell lymphoma
Gene expression profiling has been used in mature aggressive B-cell lymphomas to better define, and thus distinguish, BL and primary mediastinal large B-cell lymphoma (PMBCL) from DLBCL, because occasional overlap in morphology, immunophenotype and genetics might occur (Dave, et al 2006, Hummel, et al 2006, Rosenwald, et al 2003). The molecular classification, in fact has lead to a number of re-classifications when compared to the conventional histopathological and immunphenotypical diagnosis. Whether molecular diagnosis does translate into an advantage for the patient has never been proven because all published studies are retrospective. Nevertheless, gene expression analysis has helped to refine disease definitions and the refined definition can be easily applied in patient care. Examples of such new definitions based on gene expression profiling and translated into patient care are: (i) “double hit” lymphomas harbouring both MYC - and BCL2 - or BCL6 -translocations shown to be distinct from BL (Hummel, et al 2006); (ii) a group of lymphomas lacking MYC -translocations and aberrations in 11q share a molecular signature with BL (Salaverria, et al 2014); (iii) BCL2 protein expression might, in fact, be acceptable for the diagnosis of BL (Masque-Soler, et al 2015); and (iv) PMBCL might also occur outside of the mediastinum (Yuan, et al 2015). Using gene expression profiling to better define a disease and develop new criteria for conventional diagnostic techniques, such as morphology, immunophenotyping, FISH and targeted sequencing, might be a useful approach to translate findings into patient care. In the latter scenario, pressure regarding quality and amount of biomaterial, as well as availability and time prevent the application of sophisticated molecular technology.
Nevertheless, the transfer of gene expression based signatures into an assay that is applicable to FFPE tissue has been demonstrated previously by developing assays for molecular diagnosis and subtyping of DLBCL into the germinal centre-like (GCB) and activated B-cell like (ABC) subtypes. Immunohistochemical classifiers have an error rate that limits their application for treatment options (Meyer, et al 2011). Nevertheless, the distinction between both subtypes within DLBCL is likely to become relevant in adult DLBCL given that several drugs have been developed which might be effective in only one of the subtypes (Sehn and Gascoyne 2015). However, whether subtyping of DLBCL is going to be relevant in young patients remains controversial. In contrast to adults, DLBCL in children and adolescents is relatively homogeneous in molecular subtype, with a predominance of GCB (Oschlies, et al 2006) and have an excellent outcome using current treatment strategies. Thus, there is less pressure to introduce new subtype-specific substances into the primary therapy compared to adults. Given the high cure rates with conventional chemotherapy it seems unlikely that targeted therapy which requires molecular subtyping of DLBCL will be introduced into the primary therapy of mature aggressive B-cell lymphoma in children and adolescents. However, the poor outcome of some of these lymphomas at relapse is probably prompting physicians to order tests for molecular subtyping in order to detect possible specifically targetable pathways.
Genetic subtyping of lymphoblastic lymphoma
The development of molecular biomarkers for subgroups in T-LBL has almost exclusively been driven by translational research in paediatric lymphoma, primarily because the disease is relatively rare in adult patients. If precursor T-cell neoplasias occur in adults, they usually present as leukaemia and rarely as lymphoma. However, in children, T-LBL represents the second most common lymphoma entity. The development of biomarkers is driven by the need for risk stratification prior to initial therapy due to the high treatment-associated mortality and low survival rates for relapsed patients. Despite the fact that the World Health Organization (WHO) classification still considers T-LBL and T-cell acute lymphoblastic leukaemia (T-ALL) as one entity (Swerdlow et al 2008), there is increasing evidence that T- LBL and T-ALL differ in relevant biological features (Burkhardt, 2010). Therefore, genetic risk scores were developed specifically for T-LBL that differ from the score developed for T-ALL. One recent analysis provided evidence that the mutational status of NOTCH1/FBXW7 defined a good prognostic subgroup (Callens, et al 2012). The recently developed score by Balbach, et al (2015) defined a good-risk group (38.5% of patients with a cumulative incidence of relapse of 11±5%, NOTCH1 mutation and no RAS or PIK3–AKT pathway mutation), a high-risk group (15.4% of patients, NOTCH1 wild type in combination with PTEN mutation and loss of heterozygosityat 6q-positive patients) and an intermediate-risk group, which included patients who had neither low- nor high-risk criteria. Despite the fact that the genetically defined risk groups differ slightly between the published studies (Callens et al , 2012;Balbach et al , 2015) it seems likely that a genetic definition of risk profiles in T-LBL will guide the choice of therapy in future trials.
Advancements in novel molecular diagnostic techniques to characterize paediatric lymphomas provide unique opportunities that influence the ability to match newer agents to these new findings. However, the picture becomes more and more complex, as new sequencing technology provides insights into intratumoral heterogeneity by detection of minor subclones that will require new definitions of genotypes.
Immunological therapy in relation to paediatric non-Hodgkin lymphoma biology
Development of treatment for NHL in children and adolescents has been a paediatric oncology success story. EFS rates for NHL in children and adolescents approach 90% for mature B-NHL (BL and DLBCL), 80% for lymphoblastic lymphomas and 70% for ALCL (Minard-Colin, et al 2015). This success is mainly based on the increased understanding of lymphoma biology, description of NHL subtypes, development of risk factors for patient stratification, optimization of subtype-specific poly-chemotherapy regimens and collaboration of study groups worldwide. However, chemotherapy development has reached its limits for the main subgroups. Non-relapse mortality is close to the relapse rate in high-risk BL patients. Studies that analysed less toxic therapy for high risk BL showed an increase in relapses and lymphoma-associated death in those children treated with less intensive chemotherapy. (Cairo, et al 2007, Woessmann, et al 2005). In addition, these trials demonstrated less intensive therapy was equally effective in the standard risk group but a more dose intensive approach improved outcome in the high-risk group. Intensification of therapy did not improve survival for children with AL(Alexander, et al 2014, Le Deley,et al 2010).
These observations suggest that new treatment options need to be explored for children with NHL. One option is targeted therapies, aiming to inhibit molecular pathways crucial for lymphoma growth and survival. With unravelling of B-cellymphoma biology and B-cell receptor signalling, a whole array of targeted drugs is being developed for the treatment of mature B-NHL (Mehta-Shah and Younes 2015). However, these drugs have yet to be tested in children with NHL due to the different B NHL-subtypes in children and the very low number of relapse patients available for Phase I/II studies (e.g., Europe <15 BL relapses/year). International collaborative efforts are necessary to prioritize drugs and explore one new drug after another. It is not possible to accrue enough patients to study several drugs at the same time. In addition, new statistical approaches designed to address the small number of patients are challenges that need to be accepted by pharmaceutical companies and regulatory agencies.
One key example of targeted drugs for childhood NHL is the ALK- kinase inhibitors crizotinib and ceritinib for treatment of ALK-positive ALCL. Their development was driven by the detection of ALK-positive non-small cell lung cancers because ALK-positive ALCL is considered an orphan disease, with only 80 new paediatric cases diagnosed in Europe each year with 16 relapse events. ALK-positive ALCL may, however, be the only tumour that completely depends on activated ALK-signalling. Consequently, almost all children and adults with relapsed ALK-positive ALCL acheived a remission with crizotinib monotherapy (Gambacorti Passerini, et al 2014, Mosse, et al 2013). However, accumulating evidence suggests that ALK-inhibitors might have to be taken life-long and drug resistance is likely to develop over time. In children, a life-long therapy is difficult to accept in a disease curable in 70% of patients by five months of poly-chemotherapy. Furthermore, long-term therapy with ALK-inhibitors has a huge socio-economic impact and ALK-inhibitors have not been explored for possible late effects, especially in the growing organism. The combination of an ALK-inhibitor with chemotherapy is currently being explored in children in a randomized trial (Children's Oncology Group, ANHL12P1,ClinicalTrials.gov Identifier:NCT01979536).
These data and approaches suggest that the development of targeted therapies for childhood and adolescent NHL might be a long-lasting process that is unlikely to lead to cure for most children. Reaching a cure for paediatric NHL might be achieved by the introduction of yet another treatment option which has emerged as an increasingly powerful tool against tumours during the last decade: immunotherapy. Passive immunotherapy with the anti-CD20 antibody rituximab combined with chemotherapy is now standard of care for adults with aggressive mature B-NHL (Coiffier, et al 2002, Pfreundschuh, et al 2011) and newer antibodies continue to be explored. Even for rituximab, data in children are still limited to early clinical trials (Goldman, et al 2013, Meinhardt, et al 2010) with a conformational trial currently underway. The main reasons which inhibited the clinical development of rituximab for children include the high cure rate with standard chemotherapy, the unknown possible late effects on the developing immune system of children and the different paediatric NHL-subtypes compared to adults. The anti-CD30 immunotoxin, brentuximab vedotin, is currently licensed for the treatment of adults with relapsed Hodgkin lymphoma or ALCL. The first study in children (company-sponsored international trial C25002, ClinicalTrials.gov Identifier NCT01492088 including cases in the United States, France, Germany, Italy, Mexico, Netherlands, Spain and United Kingdom) is still enrolling patients and is challenged due to the low number of children with ALCL-relapses available. The Children’s Oncology Group is currently enrolling in a randomized Phase II front line study adding brentuximab vedotin to standard chemotherapy for ALCL (ANHL12P1, ClinicalTrials.gov Identifier: NCT01979536).
Autologous cytotoxic T-cell therapy was developed with naturally occurring anti-tumour lymphocytes in adults (Rosenberg and Restifo 2015). T cells engineered to express third generation chimeric antigen receptors (CAR) against CD19 has very successfully opened this application to children with relapsed/refractory ALL (Grupp, et al 2013, Lee, et al 2015, Maude, et al 2014) and should be further tested for the treatment of refractory B-NHL. As an intermediate between antibody and CTL, the BiTE® blinatumomab attracts T cells to CD19 positive cells. This approach – like CAR T-cells – has achieved astonishing responses in adults and some children with refractory precursor B-cell leukaemia (Hoffman and Gore 2014, Schlegel, et al 2014). However, its activity in childhood mature B-NHL is not yet known.
Active immunotherapy and stimulation of the patient´s anti-tumour immune response opens a new concept of cure for cancer in that the immune system controls a tumour and can lead to final tumour elimination. Anti-idiotype vaccination has been pursued for chronic lymphocytic leukaemia; however, its application against the highly proliferative childhood B-NHL is limited. Among childhood NHL, ALK-positive ALCL is exquisitely amenable to active immunotherapeutic approaches. Pathological, laboratory and clinical hints suggest that ALCL may provoke an immune response in patients. ALCL cells often are surrounded abundant reactive bystander T cells. Pro-inflammatory cytokines can be detected in the serum of ALCL patients at diagnosis (Mellgren, et al 2012). The high efficacy of allogeneic blood stem cell transplantation against relapsed ALCL suggests that a graft-versus ALCL effect may exist (Fukano, et al 2015, Gross, et al 2010, Strullu, et al 2015, Woessmann, et al 2006). During the 2000s, two groups described that patients with ALK-positive ALCL mount an immune response against the oncogenic ALK protein. Pulford et al (2000) first detected ALK-specific antibodies in the serum of patients with ALK-positive ALCL. ALK-reactive CD8 positive T cells were detected in the T cell repertoire of healthy persons (Passoni, et al 2002). Patients with ALK-positive ALCL mount a CD8 and CD4 positive T cell response against ALK (Ait-Tahar, et al 2007, Ait-Tahar, et al 2006, Passoni, et al 2006). The efficacy of an ALK-cDNA-based vaccination in a mouse model of ALK-positive ALCL suggests that an active immunological approach might be clinically relevant (Chiarle et al 2008). The systematic analysis of ALK-antibodies titres at diagnosis in children and adolescents with ALK-positive ALCL revealed that the titre of the ALK-antibody inversely correlates with relapse risk (Ait-Tahar, et al 2010, Mussolin, et al 2013). These findings suggest that the immune response against ALK might be implicated in the final control of human ALCL. The existence of ALCL and observation of inter-individual differences in the strength of the patient’s immune response suggest that ALCL employs immune escape mechanisms. One factor with possible therapeutic implications is the NPM1-ALK driven expression of PD-L1 (also termed PDCD1) (Marzec, et al 2008). Host factors influencing the immune response are under investigation. The observation that more girls with ALCL mount a high antibody titre compared to boys hints towards an influence of host factors in the immune response as well (Ait-Tahar, et al 2010). Current research focuses on further characterization of the patients humoral and cellular immune response against ALK as a prerequisite to develop a clinical vaccination or T cell therapy against ALK.
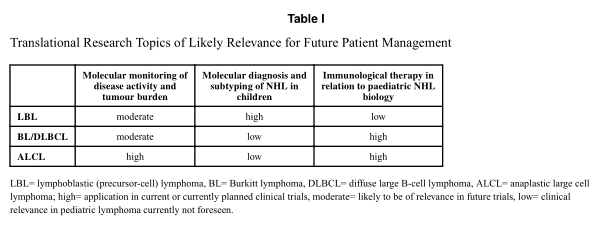
In summary, despite high survival rates reached by intensive poly-chemotherapy for children with NHL, cure for all, or at least most, children with acceptable side effects and late morbidity needs collaborative efforts of preclinical and clinical researchers. Innovative and argeted treatment options are becoming increasingly available to children and adolescents who are diagnosed with NHL. Numerous approaches combining or substituting current hemotherapy with targeted drugs and immunotherapeutic approaches need to be developed and tested clinically against NHL in children during the next decades. By influencing and translating advances in molecular characteristics of tumour presentation, therapy strategies can be developed upfront and for recurrent disease. Additional impact on improving outcome can be achieved through monitoring for MDD/MRD through unified efforts internationally. Some elements of translational research discussed in this article and at the 5th International Symposium on Childhood, Adolescent and Young Adult Non-Hodgkin Lymphoma on October 22 nd to 24 th 2015 in Varese, Italy are already very close to influencing patient management (Table I). These advances in paediatric lymphoma have been accomplished by the concerted efforts of paediatric NHL cooperative groups globally, which could be a paradigm to achieve successes in other diseases as the prognosis for childhood cancer continues to improve.
Acknowledgments
Paediatric lymphoma research of WK is supported by the Kinderkrebs-Initiative Buchholz, Holm-Seppensen (KKI).
BS is supported in part by USA NIH grant #U54MD007584. LM is supported in part by Fondazione CA.RI.PA.RO (grant#13/06). Supported in part by the Paediatric Cancer Research Foundation and Fondazione Giacomo Ascoli.
All authors performed literature search, wrote the manuscript and approved the final version for publication.
References
Agsalda M, Kusao I, Troelstrup D, Shiramizu B. Screening for residual disease in pediatric burkitt lymphoma using consensus primer pools. Adv Hematol. 2009; 2009:412163. [PubMed: 19890467]
Ait-Tahar K, Cerundolo V, Banham AH, Hatton C, Blanchard T, Kusec R, Becker M, Smith GL, Pulford K. B and CTL responses to the ALK protein in patients with ALK-positive ALCL. Int J Cancer. 2006; 118:688–695. [PubMed: 16114011] Ait-Tahar K, Barnardo MC, Pulford K. CD4 T-helper responses to the anaplastic lymphoma kinase (ALK) protein in patients with ALK positive anaplastic large-cell lymphoma. Cancer Res. 2007; 67:1898–1901. [PubMed: 17332315]
Ait-Tahar K, Damm-Welk C, Burkhardt B, Zimmermann M, Klapper W, Reiter A, Pulford K, Woessmann W. Correlation of the autoantibody response to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with tumor dissemination and relapse risk. Blood. 2010; 115:3314–3319. [PubMed: 20185586]
Alexander S, Kraveka JM, Weitzman S, Lowe E, Smith L, Lynch JC, Chang M, Kinney MC, Perkins SL, Laver J, Gross TG ,Weinstein H. Advanced stage anaplastic large cell lymphoma in children and adolescents: results of ANHL0131, a randomized phase III trial of APO versus a modified regimen with vinblastine: a report from the children's oncology group. Pediatr Blood Cancer. 2014;61:2236–2242. [PubMed: 25156886]
Balbach ST, Makarova O, Bonn BR, Zimmermann M, Rohde M, Oschlies I, Klapper W, Rossig C, Burkhardt B. Proposal of a genetic classifier for risk group stratification in pediatric T-cell lymphoblastic lymphoma reveals differences from adult T-cell lymphoblastic leukemia. Leukemia. 2015 Jul 28. 2015. [Epub ahead of print].
Basso K, Mussolin L, Lettieri A, Brahmachary M, Lim WK, Califano A, Basso G, Biondi A,
Cazzaniga G, Rosolen A. T-cell lymphoblastic lymphoma shows differences and similarities with T-cell acute lymphoblastic leukemia by genomic and gene expression analyses. Genes Chromosomes Cancer. 2011; 50:1063–1075.[PubMed:21987448]
Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013;14:e205–e217.[PubMed:23639321]
Bonn BR, Huge A, Rohde M, Oschlies I, Klapper W, Voss R, Makarova O, Rossig C, Jurgens H,Seggewiss J, Burkhardt B. Whole exome sequencing hints at a unique mutational profile of paediatric T-cell lymphoblastic lymphoma. Br J Haematol. 2015; 168:308–313. [PubMed:25160903]
Bonn BR, Rohde M, Zimmermann M, Krieger D, Oschlies I, Niggli F, Wrobel G, Attarbaschi A,Escherich G, Klapper W, Reiter A, Burkhardt B. Incidence and prognostic relevance of genetic variations in T-cell lymphoblastic lymphoma in childhood and adolescence. Blood. 2013;121:3153–3160. [PubMed: 23396305]
Burkhardt B. Paediatric lymphoblastic T-cell leukaemia and lymphoma: one or two diseases? Br J Haematol. 2010; 149:653–668. [PubMed: 19961482]
Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, Patte C, Committee FLIS. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007; 109:2736–2743. [PubMed: 17138821]
Callens C, Baleydier F, Lengline E, Ben Abdelali R, Petit A, Villarese P, Cieslak A, Minard-Colin V, Rullier A, Moreau A, Baruchel A, Schmitt C, Asnafi V, Bertrand Y, Macintyre E. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012; 30:1966–1973. [PubMed:22547598]
Cave H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, Bakkus M, Thielemans K, Grandchamp B, Vilmer E. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer--Childhood Leukemia Cooperative Group. N Engl J Med. 1998; 339:591–598. [PubMed: 9718378]
Cazzaniga G, d'Aniello E, Corral L, Biondi A. Results of minimal residual disease (MRD) evaluation and MRD-based treatment stratification in childhood ALL. Best Pract Res Clin Haematol. 2002;15:623–638. [PubMed: 12617867]
Chiarle R, Martinengo C, Mastini C, Ambrogio C, D'Escamard V, Forni G, Inghirami G. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat. Med.2008; 14:676–680. [PubMed: 18469826]
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. [PubMed: 11807147]
Coustan-Smith E, Sandlund JT, Perkins SL, Chen H, Chang M, Abromowitch M, Campana D.Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: a report from the children's oncology group. J Clin Oncol. 2009; 27:3533–3539. [PubMed: 19546402]
Damm-Welk C, Busch K, Burkhardt B, Schieferstein J, Viehmann S, Oschlies I, Klapper W,Zimmermann M, Harbott J, Reiter A, Woessmann W. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM-ALK-positive anaplastic large-cell lymphoma. Blood. 2007; 110:670–677.
[PubMed: 17392503]
Damm-Welk C, Mussolin L, Zimmermann M, Pillon M, Klapper W, Oschlies I, d'Amore ES, Reiter A,Woessmann W, Rosolen A. Early assessment of minimal residual disease identifies patients at very high relapse risk in NPM-ALK-positive anaplastic large-cell lymphoma. Blood. 2014; 123:334–337. [PubMed: 24297868]
Dave SS, Fu K, Wright GW, Lam LT, Kluin P, Boerma EJ, Greiner TC, Weisenburger DD, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Delabie J, Rimsza LM, Braziel RM, Grogan TM,Campo E, Jaffe ES, Dave BJ, Sanger W, Bast M, Vose JM, Armitage JO, Connors JM, Smeland EB, Kvaloy S, Holte H, Fisher RI, Miller TP, Montserrat E, Wilson WH, Bahl M, Zhao H, Yang L,Powell J, Simon R, Chan WC, Staudt LM, Lymphoma/Leukemia Molecular Profiling, P.Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006; 354:2431–2442. [PubMed:16760443]
Fukano R, Mori T, Kobayashi R, Mitsui T, Fujita N, Iwasaki F, Suzumiya J, Chin M, Goto H, Takahashi Y, Hara J, Park YD, Inoue M, Koga Y, Inagaki J, Sakamaki H, Adachi S, Kawa K, Kato Author Manuscript Author Manuscript Author Manuscript Author Manuscript K, Suzuki R. Haematopoietic stem cell transplantation for relapsed or refractory anaplastic large
cell lymphoma: a study of children and adolescents in Japan. Br J Haematol. 2015; 168:557–563.[PubMed: 25312752]
Gambacorti Passerini C, Farina F, Stasia A, Redaelli S, Ceccon M, Mologni L, Messa C, Guerra L,Giudici G, Sala E, Mussolin L, Deeren D, King MH, Steurer M, Ordemann R, Cohen AM, Grube M, Bernard L, Chiriano G, Antolini L, Piazza R. Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J Natl Cancer Inst. 2014; 106:djt378. [PubMed:24491302]
Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, Gross TG, Weinstein H,Bergeron S, Shiramizu B, Sanger W, Barth M, Zhi J, Cairo MS. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children's Oncology Group report. Leukemia. 2013; 27:1174–1177. [PubMed: 22940833]
Gross TG, Hale GA, He W, Camitta BM, Sanders JE, Cairo MS, Hayashi RJ, Termuhlen AM, Zhang MJ, Davies SM, Eapen M. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol Blood Marrow Transplant. 2010; 16:223–230. [PubMed: 19800015]
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B,Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013; 368:1509–1518. [PubMed: 23527958]
Hoffman LM, Gore L. Blinatumomab, a Bi-Specific Anti-CD19/CD3 BiTE((R)) Antibody for theTreatment of Acute Lymphoblastic Leukemia: Perspectives and Current Pediatric Applications.Front Oncol. 2014; 4:63. [PubMed: 24744989]
Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TF, Bernd HW, Cogliatti SB,Dierlamm J, Feller AC,Hansmann ML, Haralambieva E, Harder L, Hasenclever D, Kuhn M,Lenze D, Lichter P, Martin-Subero JI, Moller P, Muller-Hermelink HK, Ott G, Parwaresch RM,Pott C, Rosenwald A, Rosolowski M, Schwaenen C, Sturzenhofecker B, Szczepanowski M,Trautmann H, Wacker HH, Spang R, Loeffler M, Trumper L, Stein H, Siebert R. Molecular Mechanisms in Malignant Lymphomas Network Project of the Deutsche, K. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–2430. [PubMed: 16760442]
Le Deley MC, Rosolen A, Williams DM, Horibe K, Wrobel G, Attarbaschi A, Zsiros J, Uyttebroeck A,Marky IM, Lamant L, Woessmann W, Pillon M, Hobson R, Mauguen A, Reiter A, Brugieres L.Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of he randomized ALCL99-vinblastine trial. J Clin Oncol. 2010; 28:3987–3993. [PubMed:20679620]
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L,Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial.Lancet. 2015; 385:517–528. [PubMed: 25319501]
Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M,Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A. 2008; 105:20852–20857. [PubMed: 19088198]
Masque-Soler N, Szczepanowski M, Kohler CW, Aukema SM, Nagel I, Richter J, Siebert R, Spang R,Burkhardt B, Klapper W. Clinical and pathological features of Burkitt lymphoma showing expression of BCL2 - an analysis including gene expression in formalin-fixed paraffin-embedded tissue. Br J Haematol. 2015; 171:501–508. [PubMed: 26218299]
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014; 371:1507–1517. [PubMed: 25317870]
Mehta-Shah N, Younes A. Novel targeted therapies in diffuse large B-cell lymphoma. Semin Hematol. 2015; 52:126–137. [PubMed: 25805592]
Meinhardt A, Burkhardt B, Zimmermann M, Borkhardt A, Kontny U, Klingebiel T, Berthold F, Janka-Schaub G, Klein C, Kabickova E, Klapper W, Attarbaschi A, Schrappe M, Reiter A, Berlin-Frankfurt-Munster g. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin's lymphoma and Burkitt leukemia. J Clin Oncol. 2010; 28:3115–3121.[PubMed: 20516455]
Mellgren K, Hedegaard CJ, Schmiegelow K, Muller K. Plasma cytokine profiles at diagnosis in pediatric patients with non-hodgkin lymphoma. J Pediatr Hematol Oncol. 2012; 34:271–275.[PubMed: 22430582]
Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD, Ott G, Rosenwald A, Braziel RM,Campo E, Vose JM, Lenz G, Staudt LM, Chan WC, Weisenburger DD. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011; 29:200–207. [PubMed: 21135273]
Minard-Colin V, Brugieres L, Reiter A, Cairo MS, Gross TG, Woessmann W, Burkhardt B, Sandlund JT, Williams D, Pillon M, Horibe K, Auperin A, Le Deley MC, Zimmerman M, Perkins SL, Raphael M, Lamant L, Klapper W, Mussolin L, Poirel HA, Macintyre E, Damm-Welk C, Rosolen A, Patte C. Non-Hodgkin Lymphoma in Children and Adolescents: Progress Through Effective Collaboration, Current Knowledge, and Challenges Ahead. J Clin Oncol. 2015; 33:2963–2974.[PubMed: 26304908]
Mosse YP, Lim MS, Voss SD, Wilner K, Ruffner K, Laliberte J, Rolland D, Balis FM, Maris JM, Weigel BJ, Ingle AM, Ahern C, Adamson PC, Blaney SM. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol. 2013; 14:472–480. [PubMed: 23598171]
Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Semin. Oncol. 1980; 7:332–339. [PubMed:7414342]
Mussolin L, Pillon M, d'Amore ES, Conter V, Piglione M, Lo Nigro L, Garaventa A, Buffardi S, Arico M, Rosolen A. Minimal disseminated disease in high-risk Burkitt's lymphoma identifies patients with different prognosis. J Clin Oncol. 2011; 29:1779–1784. [PubMed: 21422413]
Mussolin L, Damm-Welk C, Pillon M, Zimmermann M, Franceschetto G, Pulford K, Reiter A, Rosolen A, Woessmann W. Use of minimal disseminated disease and immunity to NPM-ALK antigen to stratify ALK-positive ALCL patients with different prognosis. Leukemia. 2013;27:416–422. [PubMed: 22907048]
Mussolin L, Buldini B, Lovisa F, Carraro E, Disaro S, Nigro LL, d'Amore ES, Pillon M, Basso G.Detection and role of minimal disseminated disease in children with lymphoblastic lymphoma:The AIEOP experience. Pediatr Blood Cancer. 2015; 62:1906–1913. [PubMed: 26109265]
Neale GA, Campana D, Pui CH. Minimal residual disease detection in acute lymphoblastic leukemia:real improvement with the real-time quantitative PCR method? J Pediatr Hematol Oncol. 2003;25:100–102. [PubMed: 12571458]
Oschlies I, Klapper W, Zimmermann M, Krams M, Wacker HH, Burkhardt B, Harder L, Siebert R,Reiter A, Parwaresch R. Diffuse large B-cell lymphoma in pediatric patients belongs predominantly to the germinal-center type B-cell lymphomas: a clinicopathologic analysis of cases included in the German BFM (Berlin-Frankfurt-Munster) Multicenter Trial. Blood. 2006; 107:4047–4052. [PubMed: 16424389]
Passoni L, Scardino A, Bertazzoli C, Gallo B, Coluccia AM, Lemonnier FA, Kosmatopoulos K, Gambacorti-Passerini C. ALK as a novel lymphoma-associated tumor antigen: identification of 2 HLA-A2.1-restricted CD8+ T-cell epitopes. Blood. 2002; 99:2100–2106. [PubMed: 11877285]
Passoni L, Gallo B, Biganzoli E, Stefanoni R, Massimino M, Di Nicola M, Gianni AM, Gambacorti-Passerini C. In vivo T-cell immune response against anaplastic lymphoma kinase in patients with anaplastic large cell lymphomas. Haematologica. 2006; 91:48–55. [PubMed: 16434370]
Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, Gill DS, Walewski J, Pettengell R, Jaeger U, Zinzani PL, Shpilberg O, Kvaloy S, de Nully Brown P, Stahel R, Milpied N, Lopez-Guillermo A, Poeschel V, Grass S, Loeffler M, Murawski N. MabThera International Trial Group. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabTher International Trial (MInT) Group. Lancet Oncol. 2011; 12:1013–1022. [PubMed:21940214]
Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012; 120:1165–1174. [PubMed: 22730540]
Pulford K, Falini B, Banham AH, Codrington D, Roberton H, Hatton C, Mason DY. Immune response to the ALK oncogenic tyrosine kinase in patients with anaplastic large-cell lymphoma. Blood. 2000; 96:1605–1607. [PubMed: 10942417]
Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer.Science. 2015; 348:62–68. [PubMed: 25838374]
Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H,Delabie J, Campo E, Montserrat E, Lopez-Guillermo A, Ott G, Muller-Hermelink HK, Connors JM, Braziel R, Grogan TM, Fisher RI, Miller TP, LeBlanc M, Chiorazzi M, Zhao H, Yang L,
Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Staudt LM. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003; 198:851–862. [PubMed:12975453]
Rosolen A, Perkins SL, Pinkerton CR, Guillerman RP, Sandlund JT, Patte C, Reiter A, Cairo MS.Revised International Pediatric Non-Hodgkin Lymphoma Staging System. J Clin Oncol. 2015;33:2112–2118. [PubMed: 25940716]
Salaverria I, Bea S, Lopez-Guillermo A, Lespinet V, Pinyol M, Burkhardt B, Lamant L, Zettl A, Horsman D, Gascoyne R, Ott G, Siebert R, Delsol G, Campo E. Genomic profiling reveals different genetic aberrations in systemic ALK-positive and ALK-negative anaplastic large cell lymphomas. Br J Haematol. 2008; 140:516–526. [PubMed: 18275429]
Salaverria I, Martin-Guerrero I, Wagener R, Kreuz M, Kohler CW, Richter J, Pienkowska-Grela B, Adam P, Burkhardt B, Claviez A, Damm-Welk C, Drexler HG, Hummel M, Jaffe ES, Kuppers R, Lefebvre C, Lisfeld J, Loffler M, Macleod RA, Nagel I, Oschlies I, Rosolowski M, Russell RB,Rymkiewicz G, Schindler D, Schlesner M, Scholtysik R, Schwaenen C, Spang R, Szczepanowski M, Trumper L, Vater I, Wessendorf S, Klapper W, Siebert R. Molecular Mechanisms in Malignant Lymphoma Network, P. & Berlin-Frankfurt-Munster Non-Hodgkin Lymphoma, G. A recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphoma. Blood. 2014; 123:1187–1198. [PubMed: 24398325]
Schlegel P, Lang P, Zugmaier G, Ebinger M, Kreyenberg H, Witte KE, Feucht J, Pfeiffer M, Teltschik HM, Kyzirakos C, Feuchtinger T, Handgretinger R. Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica. 2014; 99:1212–1219. [PubMed:24727818]
Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015; 125:22–32. [PubMed: 25499448]
Swerdlow, SH.; Campo, E.; Harris, NL.; Jaffe, ES.; Pileri, SA.; Stein, H.; Thiele, J.; Vardiman, JW.
WHO classification of tumours of haematopoietic and lymphoid tissues. 4th. Lyon, France: International Agency for Research on Cancer Press; 2008.
Strullu M, Thomas C, Le Deley MC, Chevance A, Kanold J, Bertrand Y, Jubert C, Dalle JH, Paillard C, Baruchel A, Lamant L, Michel G, Brugieres L. Hematopoietic stem cell transplantation in relapsed ALK+ anaplastic large cell lymphoma in children and adolescents: a study on behalf of the SFCE and SFGM-TC. Bone Marrow Transplant. 2015; 50:795–801. [PubMed: 25822227]
Szczepanski T, Harrison CJ, van Dongen JJ. Genetic aberrations in paediatric acute leukaemias and implications for management of patients. Lancet Oncol. 2010; 11:880–889. [PubMed: 20435517]
Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, Ebell W, Escherich G, Schrappe M, Klingebiel T, Fengler R, Henze G, von Stackelberg A. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol.2010; 28:2339–2347. [PubMed: 20385996]
Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, Ludwig WD,Klingebiel T, Graf N, Gruhn B, Juergens H, Niggli F, Parwaresch R, Gadner H, Riehm H,Schrappe M, Reiter A, Group BFM. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005; 105:948–958. [PubMed: 15486066]
Woessmann W, Peters C, Lenhard M, Burkhardt B, Sykora KW, Dilloo D, Kremens B, Lang P, Fuhrer M, Kuhne T, Parwaresch R, Ebell W, Reiter A. Allogeneic haematopoietic stem cell transplantation in relapsed or refractory anaplastic large cell lymphoma of children and adolescents--a Berlin-Frankfurt-Munster group report. Br J Haematol. 2006; 133:176–182.
[PubMed: 16611309]
Yuan J, Wright G, Rosenwald A, Steidl C, Gascoyne RD, Connors JM, Mottok A, Weisenburger DD, Greiner TC, Fu K, Smith L, Rimsza LM, Jaffe ES, Campo E, Martinez A, Delabie J, Braziel RM, Cook JR, Ott G, Vose JM, Staudt LM, Chan WC. Lymphoma Leukemia Molecular Profiling, P.Identification of Primary Mediastinal Large B-cell Lymphoma at Nonmediastinal Sites by Gene
Expression Profiling. Am J Surg Pathol. 2015; 39:1322–1330. [PubMed: 26135560]