Summary Double hit lymphomas (DHL) represent a subset of highly aggressive B-cell malignancies characterized by the presence of recurrent cytogenetic rearrangements affecting MYC and either BCL2 and/or BCL6. Recent studies have expanded the concept to include MYC/BCL2 protein coexpressing lymphomas. Around 5–10% of diffuse large B-cell lymphomas are ‘double hit’ using the cytogenetic definition, whilst around 30–40% are MYC/BCL2 protein co-expressing. In this review, we provide a comprehensive overview of this condition written with the practicing clinician in mind, covering the definition and classification, when DHL should be suspected and how to make the diagnosis, the prognostic factors and a detailed discussion of recent evidence regarding optimal therapy. In particular, we discuss choice of induction regimen, the role of central nervous system-directed prophylaxis, stem cell transplantation and relapsing or refractory disease and provide our opinions based on the currently available evidence. Finally, we highlight some of the more exciting therapies currently in development for this highly aggressive disease.
Keywords: double-hit lymphoma, diffuse large B-cell lymphoma, B-cell lymphoma unclassifiable, triple-hit lymphoma, MYC. Recurrent chromosomal translocations are common in patients with B-cell malignancies (Yunis et al, 1984; Offit et al, 1991). Such translocations frequently juxtapose oncogenes with immunoglobulin loci and can be considered lymphoma-initiating events. Amongst the best studied of these is MYC; the prototypic example of an MYC-driven lymphoma is rearrangement of chromosome 8q24 in Burkitt lymphoma (BL). When MYC rearrangements occur simultaneously with other translocation partners, such as BCL2 or BCL6, the resultant lymphomas have distinctive biology and highly aggressive clinical behaviour and have been termed double hit lymphomas (DHL). Given the rarity and relatively recent awareness of this entity, prospective clinical trials have proven challenging to perform. As such, most existing data is drawn from retrospective studies or subgroup analyses from prospectively treated cohorts of patients with diffuse large B-cell lymphoma (DLBCL). The purpose of this review is to synthesize these data into a comprehensive, clinically focused overview of this challenging group of lymphomas, covering the biology, diagnostic considerations, clinical features, treatment and prognostic factors.
MYC biology and its role in lymphomas
MYC is a proto-oncogene that produces the transcription factor MYC (also termed c-Myc). Thus MYC regulates around 10% of human genes, with downstream targets influencing cellular proliferation, DNA and protein synthesis and metabolism (Mationg-Kalaw et al, 2012). Elevated expression of MYC in tumour cells can result from chromosomal translocation, gene amplification, duplication and mutations (Sewastianik et al, 2014). MYC both directly and indirectly activates CCND2 and cyclin dependent kinases (CDK) and down-regulates cell cycle inhibitors, promoting the transition from G0 to S phase (Meyer & Penn, 2008). Further, MYC drives extensive reprogramming of the microRNA transcriptome, contributing to oncogenesis (Chang et al, 2008). MYC has indispensable roles in the formation and maintenance of germinal centres (Calado et al, 2012). Paradoxically, it had also been observed that ele- vated levels of MYC can result in apoptosis (Murphy et al, 2008). Recent data suggested that MYC appears to exert cellular effects through amplification of existing transcriptionally active genes, so that its action is contextual (Lin et al, 2012; Nie et al, 2012). This recognition helps to explain the somewhat contradictory actions described above.
MYC-expressing lymphomas
Although MYC rearrangements are sine qua non in BL, their presence is not specific for this diagnosis. Some studies have described MYC rearrangements in other haematological malignancies, including B-cell lymphoma, unclassifiable with features intermediate between DLBCL and BL (BCLU), DLBCL, T-lymphoblastic lymphoma, mantle cell lymphoma, plasmablastic lymphoma, acute lymphoblastic leukaemia, follicular lymphoma and chronic lymphocytic leukaemia, the latter two usually as a secondary genetic event in the setting of histological transformation to aggressive lymphoma (Thangavelu et al, 1990; Offit et al, 1991; Karsan et al, 1993). There are key differences in the biology of BL compared with MYC-rearranged non-BL. In BL, the translocation partner in around 80% of cases is the immunoglobulin heavy chain locus (IGH) on chromosome 14q21, with less common variant translocations involving the immunoglobulin light chain kappa (IGK) and lambda (IGL) on chromosomes 2p22 and 22q11 respectively (Swerdlow et al, 2008). In contrast, in non-BL, the translocation partner is often a non-immunoglobulin gene, such as PAX5, BCL6, BCL11A or IKZF1 (Bertrand et al, 2007; Johnson et al, 2009). In BL, MYC rearrangements are often the sole abnormality; however, in DLBCL/BCLU, they usually occur as part of a complex karyotype with three or more abnormalities (Boerma et al, 2009). Furthermore, the gene expression profile in MYC-rearranged DLBCL features genes involved in the nuclear factor kappa-B pathway and antiapoptotic cascades – whilst in BL, target genes involve cellular proliferation (Hummel et al, 2006). As a consequence of these differences, MYC-driven non-BL is typically an aggressive entity with poor response to therapy and inferior prognosis. Because BL is relatively rare, in clinical
practice MYC rearrangements are encountered most frequently in the setting of DLBCL (in which 5–16% of tumours bear MYC rearrangements) and BCLU (30–50% of tumours) (Klapper et al, 2008; Savage et al, 2009; Tibiletti et al, 2009).
Defining single, double and triple hit lymphomas
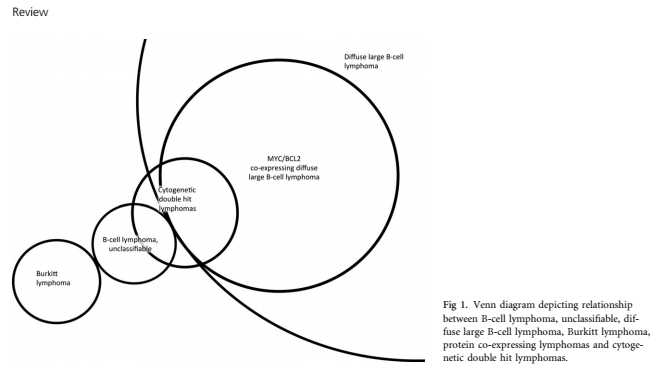
Patients with non-BL and MYC rearrangements without additional breaks in BCL2 or BCL6 are described as single hit lymphoma (SHL). These cases occur less frequently than DHL and evidence regarding the clinical relevance of the finding is mixed. SHL has been reported to confer an adverse prognosis similar to that seen in DHL, although not all studies agree on this point (Savage et al, 2009; Barrans et al, 2010; Valera et al, 2013). The German Molecular Mechanisms in Malignant Lymphoma network studied a series of patients with DHL (n = 47) and SHL (n = 31) and found similar molecular, morphological and clinical features, with no difference in survival between the two groups (Aukema et al, 2014). A Spanish group studied MYC abnormalities in 219 patients with DLBCL and found gains (19%), amplifications (2%), SHL (3%) and DHL (4%) (Valera et al, 2013). Amplifications, SHL and DHL all had negative impact on survival but MYC gains did not. However, in two other studies of patients with DLBCL, MYC breaks did not confer adverse prognosis unless BCL2 breaks were also present (Green et al, 2012a; Johnson et al, 2012). Further studies are required in larger numbers of uniformly treated patients to resolve this issue. The term ‘double hit’ was originally coined to describe lymphomas simultaneously bearing both MYC and BCL2 breaks (Thangavelu et al, 1990; Karsan et al, 1993). More broadly, the term DHL refers to B-cell lymphomas with multiple activating oncogenes, one of them being MYC. MYC/BCL2 DHL are the most common form of DHL by far, with MYC/BCL6 DHL and MYC/BCL2/BCL6 ‘triple hit’ lymphomas less frequent. In the largest series reported so far, 270 (87%) were MYC/BCL2, 16 (5%) were MYC/BCL6 and 25 (8%) were MYC/BCL2/BCL6 (Petrich et al, 2014). Rarer co-translocations involving genes such as CCND1 (BCL1), BCL3 and PAX5 have also been reported (Mitelman et al, 2014). Early publications defined DHL by cytogenetic evidence of translocations, using fluorescence in situ hybridization (FISH) (Johnson et al, 2009; Tibiletti et al, 2009; Tomita et al, 2009). DHL so defined is an aggressive disease that is typically chemo-refractory, associated with short survival and poor prognosis independent of the International Prognostic Index (IPI) (Le Gouill et al, 2007; Johnson et al, 2009; Barrans et al, 2010). Furthermore, several groups have demonstrated in large studies of patients with DLBCL that increased expression of both MYC and BCL2 protein by immunohistochemistry (hereafter referred to as MYC/BCL2 co-expressing lymphomas) have inferior survival compared with patients lacking these abnormalities – though their outcome may not be as poor as cytogenetic DHL (Johnson et al, 2012; Horn et al, 2013; Hu et al, 2013; Perry et al, 2014). A diagram representing the proposed relationship between DLBCL, BCLU, cytogenetic DHL and MYC/BCL2 co-expressing lymphomas is shown in Fig 1.
When should DHL be suspected, and when and how should patients be tested?
Due to the substantial prognostic and potential therapeutic implications of a diagnosis of DHL, rapid identification of patients is highly desirable. A key question for clinicians treating patients with lymphoma is when further testing with FISH is indicated. This question is commonly encountered in practice, as most patients with DHL have either DLBCL or BCLU, with the former being the most common non-Hodgkin lymphoma (NHL) in Western countries (Swerdlow et al, 2008). MYC rearrangements are found in up to half of patients with BCLU (Green et al, 2012b; Perry et al, 2013). MYC breaks are also identified as secondary genetic events in patients with indolent lymphoma that undergo histological transformation (Johnson et al, 2009; Tomita et al, 2009; Snuderl et al, 2010). Up to 33% of patients with immunoblastic DLBCL have MYC rearrangements (Horn et al, 2014). The prevalence of MYC/BCL2 DHL in DLBCL has been estimated at approximately 5–16% when defined by FISH, but up to one-third of patients with DLBCL are MYC/BCL2 co-expressing (Green et al, 2012a; Johnson et al, 2012; Horn et al, 2013).
An overview of studies that have reported clinical features in patients with DHL is presented in Table I. The median age of onset is 60 years, though patients as young as 17 years and as old as 87 years have been reported. The male/female ratio is approximately 2 (Oki et al, 2014; Petrich et al, 2014).
Histologically transformed indolent lymphomas account for up to 20% of cases. The median Ki67 proliferation index is around 80%, lower than that seen in BL (typically 95–100%). Features of high tumour burden, such as advanced stage, raised serum lactate dehydrogenase (LDH), B symptoms and bone marrow involvement are all common at diagnosis. Unfortunately, these features either alone or in combination are not reliable indicators of underlying DHL. The lack of consistent clinical findings indicating which patients might be harbouring DHL has led some investigators to conclude that all patients with DLBCL should be investigated with FISH for MYC breaks (Landsburg et al, 2014). However, FISH requires specialized equipment and technical expertise and is time consuming and costly. Nuclear MYC protein staining by immunohistochemistry (hereafter referred to as MYC-IHC) is comparatively cheaper and becoming widely available in routine diagnostic laboratories; reproducibility is high, at least in large academic centres with reporting expertise (Johnson et al, 2012). In a resource-constrained environment it is therefore relevant to ask whether MYC-IHC can be used to screen patients for FISH testing, as a commercially available antibody exists (clone Y69 Epitomics, Burlingame, CA, USA). The studies addressing this specific question have shown that MYC rearrangements correlate highly with MY protein expression (Tapia et al, 2011; Green et al, 2012b; Kluk et al, 2012; Horn et al, 2013). Horn et al (2014) examined the impact of MYC, BCL2 and BCL6 breaks (by FISH) and protein expression (by IHC) within a subset of patients with DLBCL treated on the RICOVER-60 (Rituximab with CHOP [cyclophosphamide, daunorubicin, Oncovin, prednisone] over 60 Years) study. Of a total of 949 patients, FISH data was available in 442 (47%) and MYC staining by IHC was interpretable in 283 (30%). Amongst MYC-rearranged cases, 18/26 (69%) were positive by IHC using a cut-off of ≥40% compared with 67/241 (28%) of non-rearranged cases. In this dataset, using IHC to screen patients for FISH testing, 8 (31%) cases of MYC/BCL2 DHL would have been missed. In contrast, Green et al (2012b) studied 205 DLBCL biopsy specimens by both IHC (using the same antibody) and FISH and determined that ≥70% MYC-IHC+ lymphoma cells was the optimal cut-off, resulting in 100% sensitivity and 93% specificity for the presence of MYC breaks. Taken together, these studies demonstrate that MYC-IHC has high sensitivity and potential as a screening test for MYC breaks, however the optimal threshold remains to be defined.
Recommendation
All patients with DLBCL should be tested for MYC and BCL2 by IHC, as their presence defines protein co-expressing lymphoma. Ideally, all patients with DLBCL would have FISH testing for MYC rearrangements. A compromise would be to use MYC-IHC to screen patients for further testing with FISH. Although the precise cut-off is uncertain, we propose a value of MYC-IHC ≥30% as a trigger to perform FISH, to minimize false negatives. Patients found to have MYC rearrangements should have subsequent FISH for BCL2 and BCL6 rearrangements. All patients with BCLU, immunoblastic DLBCL and transformed indolent lymphomas should have FISH for MYC rearrangements.
Are MYC/BCL2 co-expressing lymphomas the same as FISH-defined MYC/BCL2 DHL?
Patients with DLBCL whose tumours express both MYC/BCL2 co-expressing lymphomas are more common than cytogenetic DHL, as mechanisms other than translocation (such as amplification, mutation and microRNA-dependent mechanisms) can result in over-expression of these proteins
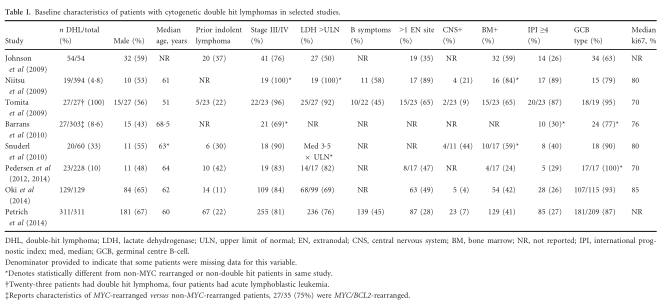
(Ott et al, 2013). MYC/BCL2 co-expressing lymphomas account for 21–34% of DLBCL; their outcomes appear inferior to non-overexpressing cases but not as poor as cytogenetic MYC/BCL2 DHL (Green et al, 2012a,b; Johnson et al, 2012; Kluk et al, 2012; Hu et al, 2013; Valera et al, 2013; Fiskvik et al, 2014; Perry et al, 2014; Yan et al, 2014; Zhou et al, 2014) (Fig 2). The precise cut-off for a positive result has varied slightly between studies, but ≥40% for MYC-IHC and ≥70% for BCL2-IHC are typical (Green et al, 2012a; Johnson et al, 2012; Hu et al, 2013). Whether overexpression of MYC, BCL2 or BCL6 in isolation is adversely prognostic remains a subject of debate due to inconsistencies in the existing literature. In several studies, over-expression of MYC or BCL2 protein has not had a negative impact on survival (Green et al, 2012a; Johnson et al, 2012; Hu et al, 2013), however, in other studies, over-expression of MYC, BCL2 or BCL6 protein was adversely prognostic (Horn et al, 2013;
Visco et al, 2013; Tzankov et al, 2014). Further, in contrast to cytogenetic MYC/BCL2 DHL (which is usually germinal centre B-cell origin) 63–76% of MYC/BCL2 co-expressing lymphomas have activated B-cell origin (Green et al, 2012a; Johnson et al, 2012; Hu et al, 2013).
What are the prognostic factors for patients with DHL?
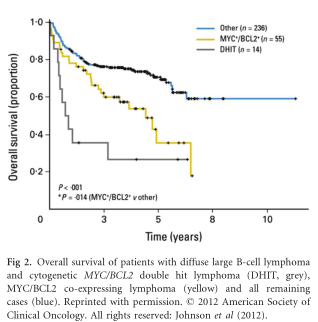
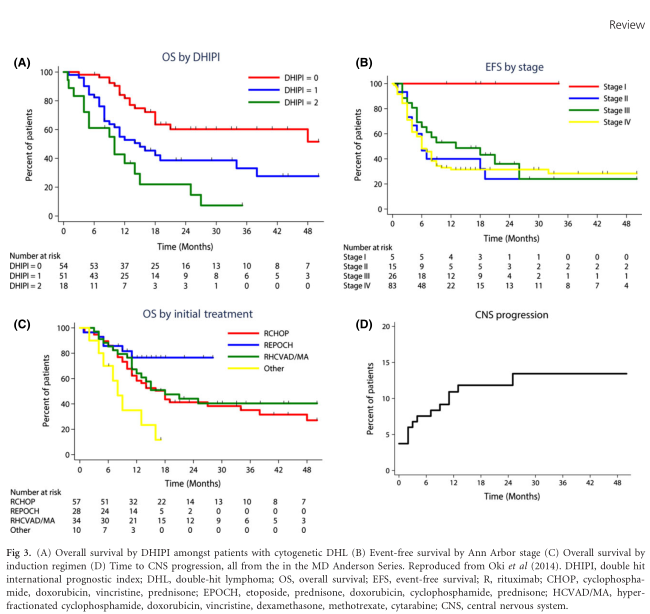
Patients with cytogenetic MYC/BCL2 DHL have an aggressive course with poorer responses to standard therapies and shorter progression-free (PFS) and overall survival (OS) compared with MYC germline DLBCL, independent from clinical risk factors (Klapper et al, 2008; Johnson et al, 2009). The IPI has some prognostic relevance in DHL but limited discriminatory power, as elevated LDH, multiple extranodal sites of involvement and advanced stage are common – therefore most are categorized as high-risk (Petrich et al, 2014). Several other potential prognostic factors have been explored, however, given almost all reported series have been small, single centre and retrospective, findings have been somewhat inconsistent. Nonetheless, some common themes emerge. Two studies have found DHL patients with a MYC-IGH translocation partner (compared with non-immunoglobulin) to be associated with an adverse prognosis (Johnson et al, 2009; Pedersen et al, 2014). One of these studies also identified BCLU morphology as an adverse prognostic factor, however these patients were also more likely to have non immunoglobulin translocation partners and marrow involvement (Johnson et al, 2009). Oki et al (2014) reported the outcomes of 129 patients with cytogenetic DHL treated at MD Anderson Cancer Center (MDACC) anddeveloped a double-hit IPI (DHIPI) using two variables independently significant for adverse OS: performance status ≥2 and bone marrow involvement (Fig 3A). Non-MYC translocation (BCL2, BCL6 or triple-hit lym-phoma) and morphology (DLBCL or BCLU) had no influence on survival. Interestingly, the limited number of patients with stage I disease (n = 5) and grade 3B follicular lymphoma (n = 2) were all alive and free from progression at the time of reporting. Patients with stages II–IV did not have significantly different outcomes (Fig 3B) (Oki et al, 2014). A large multi-centre retrospective collaboration between 23 US academic centres reporting on 311 patients with cytogenetic DHL found white blood cell count >10 9 10 9 /l, LDH ≥ 39 upper limit of normal, stage III/IV and the presence of central nervous system (CNS) involvement to be prognostic (Petrich et al, 2014).
What is the optimal treatment strategy for patients with DHL?
In this section we describe the currently available evidence informing treatment decisions for patients with DHL. A summary of selected studies is presented in Table II. It is noteworthy that most data are derived from retrospective series and, as such, are unavoidably limited by selection bias. We then provide our opinion on how these data should be translated in to clinical practice.
Choice of induction regimen
Patients with DHL have poor outcomes using standard therapy for DLBCL. In the MDACC series of patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP), only 23/57 (40%) achieved a complete response (CR). The 2-year PFS and OS rates were 25% and 41%, respectively (Oki et al, 2014). These disappointing figures are mirrored in other studies, with induction regimen, found that use of intensive induction improved OS compared with R-CHOP [HR = 0?53 (95% CI 0?29–0?98, P = 0?042] (Petrich et al, 2014). A meta-analysis of 401 patients also reported PFS advantage in favour of DA-EPOCH-R (n = 91) over R-CHOP (n = 180) (HR 0?64, 95% CI 0?42–0?92). In this analysis, R-HCVAD or R-COD-OXMIVAC induction (combined n = 130) were not associated with prolongation of PFS, and no induction regimens were associated with improved OS (Howlett et al, 2014). Pre-liminary results from a prospective multicentre phase II study in 52 patients with MYC-rearranged DLBCL/BCLU (including 14 with BCL2-rearrangements) showed that, after a median follow up of 14 months, the PFS was an encourag-ing 87% (Dunleavy et al, 2014).
Recommendation
Because of the suboptimal outcomes of patients with DHL, all patients should be encouraged to enrol on prospective clinical trials where possible. Outside this setting, we recommend patients with DHL and suitable performance status and organ function receive dose adjusted DA-EPOCH-R.
Patients unfit for intensive induction therapies and ineligible for trials may be treated with R-CHOP, though the probability of disease control and survival beyond 2 years is minimal.
What is the risk of CNS involvement in patients with DHL?
Cytogenetic DHL appears to have a propensity for CNS involvement. Early, smaller series suggested the risk was markedly increased (Kanungo et al, 2006; Le Gouill et al, 2007; Snuderl et al, 2010). However, in the two largest retrospective series (Oki et al, 2014; Petrich et al, 2014), 4–7% of patients had CNS involvement at baseline. In the MDACC series, the 3-year cumulative risk of CNS involvement was 13%; with the only predictive factor identified being DHIPI score [HR 2?14 (95% CI 1?08–4?22), P = 0?029] (Fig 3D). Amongst CNS-negative patients at diagnosis, those who received intrathecal methotrexate experienced a lower rate of CNS progression (3-year incidence 5% vs. 15%, P = 0?017) (Oki et al, 2014). Petrich et al (2014) did not specifically report on rates of CNS progression, but patients who received CNS-directed prophylaxis had improvement in OS. However, both analyses are limited by their retrospective nature and potential for bias. Savage et al (2014) studied 447 patients with DLBCL; 131 (29%) were MYC/BCL2 co-expressing and this finding was a significant independent predictor of CNS involvement (HR 3?76, P = 0?007). Furthermore, the 2-year CNS-progression risk in patients with MYC/BCL2 co-expressing lymphomas and IPI 2–3 and 4–5 was 12?6% and 17?2% respectively, significantly higher than patients not co-expressing these proteins (Savage et al, 2014).
Recommendation
Patients with cytogenetic DHL should receive CNS-directed prophylaxis as part of their induction therapy. Our current policy is to give intrathecal methotrexate once per cycle. In DLBCL, the addition of 2–4 cycles of systemic high dose methotrexate at the completion of chemoimmunotherapy lowers risk of CNS-progression further; this could be considered in patients not receiving this as part of induction therapy (Cheah et al, 2014; Ferreri et al, 2014). Patients with MYC/BCL2 co-expressing lymphoma and IPI ≥2 should receive also CNS-directed prophylaxis (Savage et al, 2014).
Role of transplant
The role of autologous (auSCT) and/or allogeneic (alloSCT) stem cell transplantation in DHL remains unclear. It should be noted that the data addressing this issue are limited by guarantee-time bias in favour of patients surviving long enough to receive stem cell transplantation. In the MDACC series, 26 (20%) of patients had frontline transplantation, including three patients who received alloSCT. Most of this cohort comprised patients receiving induction with DA-EPOCH-R (n = 14, 54%). Despite these patients being selected by virtue of achieving an initial response to induction, and having adequate organ function and performance status, frontline transplant was not associated with a statistically significant prolongation of either EFS or OS (Oki et al, 2014). The US multi-centre collaboration also examined the impact of high dose therapy and stem cell rescue in patients in CR after induction therapy; they also reported no significant improvement in OS for those who received transplantation (n = 39: auSCT 28, alloSCT 11) compared with those observed (n = 112) (P = 0?14) (Petrich et al, 2014). A subset analysis of 16 patients with MYC/BCL2 protein co-expressing lymphoma in the Southwestern Oncology Group (SWOG) S9704 study (in which patients with aggressive NHL received induction with CHOP ? R with randomization to auSCT or observation) was presented. After a median 127 months of follow up, auSCT was associated with a median PFS of 41 vs. 11 months in patients not consolidated. Only three patients in this study had MYC/BCL2 cytogenetic DHL and their outcomes were poor (Puvvadda et al, 2014).
Recommendation
Acknowledging the limitations of the data, auSCT may be considered for patients with cytogenetic DHL and protein co-expressing lymphoma in CR following induction therapy, ideally in the setting of a prospective clinical trial. AlloSCT is currently unsupported by data in DHL, and is not recommended.
Role of radiation
Given that patients with DHL usually present with advanced stage disease and therapeutic failure is usually due to primary chemo-refractoriness or systemic disease progression, radiotherapy has a limited role. The empiric use of radiation may be reasonable in patients with advanced stage disease in selected circumstances, such as spinal cord compression, symptom palliation, hypermetabolic residual masses following completion of chemotherapy or sanctuary sites, such as the testis. In the MDACC series 12 patients (9%) received radiation as part of their initial therapy and the impact on outcome was not assessed due to the limited numbers of patients.
Relapsed and refractory disease
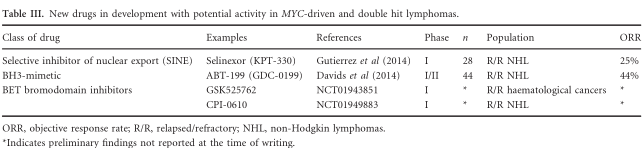
With currently available therapies, the outcome of patients with DHL who either fail to respond to induction or progress after initial response is particularly bleak. Amongst 79 such patients in the MDACC series, the 12-month post-progression survival was 20%, with only two patients (3%) remaining alive beyond 2 years (Oki et al, 2014). Petrich Table III. New drugs in development with potential activity in MYC-driven and double hit lymphomas. Class of drug Examples References Phase n Population ORR Selective inhibitor of nuclear export (SINE) Selinexor (KPT-330) Gutierrez et al (2014) I 28 R/R NHL 25% BH3-mimetic ABT-199 (GDC-0199) Davids et al (2014) I/II 44 R/R NHL 44% BET bromodomain inhibitors GSK525762 NCT01943851 I * R/R haematological cancers * CPI-0610 NCT01949883 I * R/R NHL * ORR, objective response rate; R/R, relapsed/refractory; NHL, non-Hodgkin lymphomas. *Indicates preliminary findings not reported at the time of writing. et al (2014) reported that, amongst patients who received salvage therapy – typically rituximab, ifosfamide, carboplatin and etoposide (R-ICE) – the median post-progression survival was 17 months.
Recommendation
Given the disastrous consequences of progression, we recommend patients with DHL who wish to receive further therapy be enrolled into prospective investigational protocols evaluating novel agents with either sound preclinical rationale or demonstrated activity in MYC-driven lymphomas. In clinically appropriate patients, use of a non-cross resistant regimen, such as rituximab, dexamethasone, cytarabine and cisplatin (R-DHAP) or R-ICE (in etoposide-na€ıve patients) may be considered to bridge patients to enrolment on clinical trials or as part of an active palliative approach.
Novel approaches
Given that most patients with DHL will die rapidly from their disease, development of new and effective agents is a significant unmet medical need. MYC-driven lymphomas are the subject of much research attention, with several candidate therapies in early phase clinical trials. Selinexor (KPT-330) is a first-in-class selective inhibitor of nuclear export which binds to the nuclear export protein XPO1, forcing nuclear retention and activation of tumour suppressor proteins (Parikh et al, 2014). It has shown promising preclinical activity in a range of haematological cancers (Etchin et al, 2013; Walker et al, 2013; Zhong et al, 2014). Preliminary phase I data in patients with relapsed lymphomas (including four patients with DHL) suggest promising single agent activity with negligible toxicity (Gutierrez et al, 2014). This drug was recently designated orphan drug status by the US Food and Drug Administration and further efficacy studies in this promising compound are anticipated with interest. BET bromodomain inhibitors have been reported to interfere with MYC-inducing cell differentiation, cell cycle inhibition and pro-apoptotic activity (Delmore et al, 2011). Several compounds in this class have shown preclinical activity and are in early clinical development (Filippakopoulos & Knapp, 2014). The anti-apoptotic protein BCL2 mediates chemoresistance in lymphoma cells, and is a rational target in DHL. ABT-199 is a small molecule, orally administered BH3-mimetic that has shown promising single-agent activity in heavily pre-treated NHL (Davids et al, 2014). ABT-199 is currently being explored with bendamustine and rituximab in a phase I study of patients with relapsed NHL, including patients with double hit lymphoma (NCT01594229). Lenalidomide and ibrutinib have activity in DLBCL, but studies have not specified the MYC/BCL2 status of patients treated (Witzig et al, 2011; Zinzani et al, 2011; Vose et al, 2013; Wang et al, 2013; Vitolo et al, 2014; Younes et al, 2014). A summary of selected investigational agents holding promise in double hit lymphomas is presented in Table III.
Conclusion
DHL remains a challenging problem for clinicians and patients alike. Clinicians should be proactive in requesting FISH for MYC breaks in patients with DLBCL, BCLU and transformed indolent lymphomas as a timely diagnosis to facilitate the use of DA-R-EPOCH and CNS-directed pro- phylaxis. Ultimately, only patient and physician participation in ongoing clinical trial efforts will result in improvement in outcomes for patients with this highly aggressive form of NHL.
Contributions
CYC performed the literature review and wrote the first draft of the manuscript. FT designed the research and co-wrote the manuscript. YO and JRW reviewed and co-wrote the manuscript.
References
Aukema, S.M., Kreuz, M., Kohler, C.W., Rosolowski, M., Hasenclever, D., Hummel, M., Kuppers, R., Lenze, D., Ott, G., Pott, C., Richter, J., Ro- senwald, A., Szczepanowski, M., Schwaenen, C., Stein, H., Trautmann, H., Wessendorf, S., Trumper, L., Loeffler, M., Spang, R., Kluin, P.M., Klapper, W., Siebert, R. & Molecular Mechanisms in Malignant Lymphomas Network Project. (2014) Biological characterization of adult MYC-translocation-positive mature B-cell lymphomas other than molecular Burkitt lymphoma. Haematologica, 99, 726–735.
Barrans, S., Crouch, S., Smith, A., Turner, K., Owen, R., Patmore, R., Roman, E. & Jack, A. (2010) Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. Journal of Clinical Oncology, 28, 3360–3365.
Bertrand, P., Bastard, C., Maingonnat, C., Jardin, F., Maisonneuve, C., Courel, M.N., Ruminy, P., Picquenot, J.M. & Tilly, H. (2007) Mapping of MYC breakpoints in 8q24 rearrangements involving non-immunoglobulin partners in B-cell lymphomas. Leukemia, 21, 515–523.
Boerma, E.G., Siebert, R., Kluin, P.M. & Baudis, M. (2009) Translocations involving 8q24 in Burkitt lymphoma and other malignant lymphomas: a historical review of cytogenetics in the light of todays knowledge. Leukemia, 23, 225–234.
Calado, D.P., Sasaki, Y., Godinho, S.A., Pellerin, A., Kochert, K., Sleckman, B.P., de Alboran, I.M., Janz, M., Rodig, S. & Rajewsky, K. (2012) The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nature Immunology, 13, 1092–1100.
Chang, T.C., Yu, D., Lee, Y.S., Wentzel, E.A., Arking, D.E., West, K.M., Dang, C.V., Thomas Tikhonenko, A. & Mendell, J.T. (2008) Widespread microRNA repression by Myc contributes to tumorigenesis. Nature Genetics, 40, 43–50.
Cheah, C.Y., Herbert, K.E., O’Rourke, K., Kennedy, G.A., George, A., Fedele, P.L., Gilbertson, M., Tan, S.Y., Ritchie, D.S., Opat, S.S., Prince, H.M., Dickinson, M., Burbury, K., Wolf, M., Januszewicz, E.H., Tam, C.S., Westerman, D.A., Carney, D.A., Harrison, S.J. & Seymour, J.F. (2014) A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. British Journal of Cancer, 111,1072–1079.
Davids, M.S., Seymour, J.F., Gerecitano, J.F., Kahl, B.S., Pagel, J.M., Wierda, W.G., Anderson, M.A., Rudersdorf, N., Gressick, L.A., Montalvo, N.P., Yang, J., Zhu, M., Dunbar, M., Cerri, E., Enschede, S.H., Humerickhouse, R. & Roberts, A.W. (2014) Phase I study of ABT-199 (GDC-0199)
in patients with relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL): responses observed in diffuse large B-cell (DLBCL) and follicular lymphoma (FL) at higher cohort doses. Journal of Clinical Oncology (ASCO Meeting Abstracts), 32, 8522.
Delmore, J.E., Issa, G.C., Lemieux, M.E., Rahl, P.B., Shi, J., Jacobs, H.M., Kastritis, E., Gilpatrick, T., Paranal, R.M., Qi, J., Chesi, M., Schinzel, A.C., McKeown, M.R., Heffernan, T.P., Vakoc, C.R., Bergsagel, P.L., Ghobrial, I.M., Richardson, P.G., Young, R.A., Hahn, W.C., Anderson, K.C., Kung, A.L., Bradner, J.E. & Mitsiades, C.S. (2011) BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell, 146, 904–917.
Dunleavy, K., Fanale, M., LaCasce, A., Noy, A., Caimi, P.F., Parekh, S., Hayslip, J.W., Jagadeesh, D., Lord, R.S., Lechowicz, M.J., Gaur, R., Lucas,
A., Staudt, L.M., Steinberg, S.M., Kahl, B.S., Friedberg, J.W., Little, R.F., Bartlett, N.L. & Wilson, W.H. (2014) Preliminary Report of a Multicenter Prospective Phase II Study of DAEPOCH-R in MYC-Rearranged Aggressive B-Cell Lymphoma In: ASH Annual Meeting Abstracts. In press. Etchin, J., Sanda, T., Mansour, M.R., Kentsis, A., Montero, J., Le, B.T., Christie, A.L., McCauley, D., Rodig, S.J., Kauffman, M., Shacham, S., Stone, R., Letai, A., Kung, A.L. & Thomas Look, A. (2013) KPT-330 inhibitor of CRM1 (XPO1)mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. British Journal of Haematology, 161, 117–127.
Ferreri, A.J., Bruno-Ventre, M., Donadoni, G., Ponzoni, M., Citterio, G., Foppoli, M., Vignati, A., Scarfo, L., Sassone, M., Govi, S. & Caligaris Cappio, F. (2014) Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. British Journal of Haematology. DOI: 10.1111/bjh.13194 [Epub ahead of print].
Filippakopoulos, P. & Knapp, S. (2014) Targeting bromodomains: epigenetic readers of lysine acetylation. Nature Reviews. Drug Discovery, 13,
337–356.
Fiskvik, I., Beiske, K., Delabie, J., Yri, O., Spetalen, S., Karjalainen-Lindsberg, M.L., Leppa, S., Liestol, K., Smeland, E.B. & Holte, H. (2014) Com-
bining MYC, BCL-2 and TP53 gene and protein expression alterations improves risk stratification in diffuse large B-cell lymphoma. Leukaemia &
Lymphoma, 1–18. [Epub ahead of print].
Green, T.M., Young, K.H., Visco, C., Xu-Monette, Z.Y., Orazi, A., Go, R.S., Nielsen, O., Gadeberg, O.V., Mourits-Andersen, T., Frederiksen, M., Pedersen, L.M. & Moller, M.B. (2012a) Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of Clinical Oncology, 30, 3460–3467.
Green, T.M., Nielsen, O., de Stricker, K., Xu-Monette, Z.Y., Young, K.H. & Moller, M.B. (2012b) High levels of nuclear MYC protein predict the presence of MYC rearrangement in diffuse large B-cell lymphoma. American Journal of Surgical Pathology, 36, 612–619.
Gutierrez, M., Goy, A., Byrd, J.C., Flynn, J.M., Sorensen, M., Brown, P., Gabrail, N.Y., Savona, M., Flinn, I., Baz, R.C., Shah, B.D., Stone, R.M., Jacobsen, E., Kukreti, V., Tiedemann, R.E., Rashal, T., Mirza, M.R., Shacham, S., Kauffman, M. & Kuruvilla, J. (2014) A phase 1 dose-escalation study of the oral selective inhibitor of nuclear export (SINE) KPT-330 (selinexor) in patients (pts) with heavily pretreated non-Hodgkin lymphoma (NHL). Journal of Clinical Oncology (ASCO Meeting Abstracts), 32, 8518.
Horn, H., Ziepert, M., Becher, C., Barth, T.F., Bernd, H.W., Feller, A.C., Klapper, W., Hummel, M., Stein, H., Hansmann, M.L., Schmelter, C., Moller, P., Cogliatti, S., Pfreundschuh, M., Schmitz, N., Trumper, L., Siebert, R., Loeffler, M., Rosenwald, A., Ott, G. & German HighGrade Non-Hodgkin Lymphoma Study Group. (2013) MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood, 121, 2253–2263.
Horn, H., Staiger, A.M., Vohringer, M., Hay, U., Campo, E., Rosenwald, A., Ott, G. & Ott, M.M. (2014) Diffuse Large B-cell Lymphomas of Im-munoblastic Type Are a Major Reservoir for MYC-IGH Translocations. American Journal of Surgical Pathology. DOI 10.1097/PAS.00000 00000000319 [Epub ahead of print].
Howlett, C., Landsburg, D.J., Chong, E., Snedecor, S.J., Schuster, S., Green, T.M., Cohen, J.B., Svoboda, J., Nasta, S., Feldman, T., Rago, A., Land, D., Walsh, K.M., Goy, A. & Mato, A.R. (2014) Front-line Dose-Escalated Immunochemotherapy Is Associated with a Significant PFS (but not OS) Advantage in 401 patients with Double-Hit Lymphomas: A Systematic Review and Meta-Analysis.
In: ASH Annual Meeting Abstracts. In press. Hu, S., Xu-Monette, Z.Y., Tzankov, A., Green, T., Wu, L., Balasubramanyam, A., Liu, W.M., Visco, C., Li, Y., Miranda, R.N., Montes-Moreno, S., Dybkaer, K., Chiu, A., Orazi, A., Zu, Y., Bhagat, G., Richards, K.L., Hsi, E.D., Choi, W.W., Zhao, X., van Krieken, J.H., Huang, Q., Huh, J., Ai, W., Ponzoni, M., Ferreri, A.J., Zhou, F., Slack, G.W., Gascoyne, R.D., Tu, M., Variakojis, D., Chen, W., Go, R.S., Piris, M.A., Moller, M.B., Medeiros, L.J. & Young, K.H. (2013) MYC/BCL2 protein coexpression contributes to the inferior survival of activated
B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood, 121, 4021–4031; quiz 4250.
Hummel, M., Bentink, S., Berger, H., Klapper, W., Wessendorf, S., Barth, T.F., Bernd, H.W., Cogliatti, S.B., Dierlamm, J., Feller, A.C., Hansmann,
M.L., Haralambieva, E., Harder, L., Hasenclever, D., Kuhn, M., Lenze, D., Lichter, P., MartinSubero, J.I., Moller, P., Muller-Hermelink, H.K., Ott, G., Parwaresch, R.M., Pott, C., Rosenwald, A., Rosolowski, M., Schwaenen, C., Sturzenhofecker, B., Szczepanowski, M., Trautmann, H., Wacker, H.H., Spang, R., Loeffler, M., Trumper, L., Stein, H., Siebert, R. & Molecular Mechanisms in Malignant Lymphomas Network Project of the Deutsche Krebshilfe. (2006) A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. New England Journal of Medicine, 354, 2419–2430.
Johnson, N.A., Savage, K.J., Ludkovski, O., BenNeriah, S., Woods, R., Steidl, C., Dyer, M.J., Siebert, R., Kuruvilla, J., Klasa, R., Connors, J.M., Gascoyne, R.D. & Horsman, D.E. (2009) Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood, 114, 2273–2279.
Johnson, N.A., Slack, G.W., Savage, K.J., Connors, J.M., Ben-Neriah, S., Rogic, S., Scott, D.W., Tan, K.L., Steidl, C., Sehn, L.H., Chan, W.C., Iqbal, J., Meyer, P.N., Lenz, G., Wright, G., Rimsza, L.M., Valentino, C., Brunhoeber, P., Grogan, T.M., Braziel, R.M., Cook, J.R., Tubbs, R.R., Weisenburger, D.D., Campo, E., Rosenwald, A., Ott, G., Delabie, J., Holcroft, C., Jaffe, E.S., Staudt, L.M. & Gascoyne, R.D. (2012) Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of Clinical Oncology, 30, 3452–3459.
Kanungo, A., Medeiros, L.J., Abruzzo, L.V. & Lin, P. (2006) Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC transloca- tion generally have a poor prognosis. Modern Pathology, 19, 25–33.
Karsan, A., Gascoyne, R.D., Coupland, R.W., Shep- herd, J.D., Phillips, G.L. & Horsman, D.E. (1993) Combination of t(14;18) and a Burkitt’s type translocation in B-cell malignancies. Leukaemia & Lymphoma, 10, 433–441.
Klapper, W., Stoecklein, H., Zeynalova, S., Ott, G., Kosari, F., Rosenwald, A., Loeffler, M., Trumper, L., Pfreundschuh, M., Siebert, R. & German High-Grade Non-Hodgkin’s Lymphoma Study Group. (2008) Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B-cell lymphomas treated within randomized trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Leukemia, 22, 2226–2229.
Kluk, M.J., Chapuy, B., Sinha, P., Roy, A., Dal Cin, P., Neuberg, D.S., Monti, S., Pinkus, G.S., Shipp, M.A. & Rodig, S.J. (2012) Immunohisto-chemical detection of MYC-driven diffuse large B-cell lymphomas. PLoS One, 7, e33813.
Landsburg, D.J., Nasta, S.D., Svoboda, J., MorrisLette, J.J. & Schuster, S.J. (2014) ‘Double-Hit’ cytogenetic status may not be predicted by base-
line clinicopathological characteristics and is highly associated with overall survival in B cell lymphoma patients. British Journal of Haematology, 166, 369–374.
Le Gouill, S., Talmant, P., Touzeau, C., Moreau, A., Garand, R., Juge-Morineau, N., Gaillard, F., Gastinne, T., Milpied, N., Moreau, P., Harous- seau, J.L. & Avet-Loiseau, H. (2007) The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rear-
rangement. Haematologica, 92, 1335–1342.
Lin, C.Y., Loven, J., Rahl, P.B., Paranal, R.M., Burge, C.B., Bradner, J.E., Lee, T.I. & Young, R.A. (2012) Transcriptional amplification in tumor cells with elevated c-Myc. Cell, 151, 56–67.
Mationg-Kalaw, E., Tan, L.H., Tay, K., Lim, S.T., Tang, T., Lee, Y.Y. & Tan, S.Y. (2012) Does the proliferation fraction help identify mature B cell
lymphomas with double- and triple-hit translocations? Histopathology, 61, 1214–1218.
Meyer, N. & Penn, L.Z. (2008) Reflecting on 25 years with MYC. Nature Reviews Cancer, 8, 976–990.
Mitelman, F., Johansson, B. & Mertens, F. (Eds.) (2014) Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. http:// cgap.nci.nih.gov/Chromosomes/Mitelman.
Murphy, D.J., Junttila, M.R., Pouyet, L., Karnezis, A., Shchors, K., Bui, D.A., Brown-Swigart, L., Johnson, L. & Evan, G.I. (2008) Distinct thresh- olds govern Myc’s biological output in vivo. Cancer Cell, 14, 447–457.
Nie, Z., Hu, G., Wei, G., Cui, K., Yamane, A., Resch, W., Wang, R., Green, D.R., Tessarollo, L., Casellas, R., Zhao, K. & Levens, D. (2012) c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell, 151, 68–79.
Niitsu, N., Okamoto, M., Miura, I. & Hirano, M. (2009) Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t (14;18) and 8q24/c-MYC translocations. Leukemia, 23, 777–783.
Offit, K., Jhanwar, S.C., Ladanyi, M., Filippa, D.A. & Chaganti, R.S. (1991) Cytogenetic analysis of 434 consecutively ascertained specimens of non-Hodgkin’s lymphoma: correlations between recurrent aberrations, histology, and exposure to cytotoxic treatment. Genes Chromosomes Cancer, 3, 189–201.
Oki, Y., Noorani, M., Lin, P., Davis, R.E., Neelapu, S.S., Ma, L., Ahmed, M., Rodriguez, M.A., Hagemeister, F.B., Fowler, N., Wang, M., Fanale, M.A., Nastoupil, L., Samaniego, F., Lee, H.J., Dabaja, B.S., Pinnix, C.C., Medeiros, L.J., Nieto, Y., Khouri, I., Kwak, L.W., Turturro, F., Ro-maguera, J.E., Fayad, L.E. & Westin, J.R. (2014) Double hit lymphoma: the MD Anderson Cancer Center clinical experience. British Journal of Haematology, 166, 891–901.
Ott, G., Rosenwald, A. & Campo, E. (2013) Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Hematology/The Education Program of the American Society of Hematology, 2013, 575–583.
Parikh, K., Cang, S., Sekhri, A. & Liu, D. (2014)
Selective inhibitors of nuclear export (SINE)- a novel class of anti-cancer agents. Journal of Hematology & Oncology, 7, 78.
Pedersen, M.O., Gang, A.O., Poulsen, T.S., Knudsen, H., Lauritzen, A.F., Nielsen, S.L., Gang, U.O. & Norgaard, P. (2012) Double-hit BCL2/MYC translocations in a consecutive cohort of patients with large B-cell lymphoma – a single centre’s experience. European Journal of Haematology, 89, 63–71.
Pedersen, M.O., Gang, A.O., Poulsen, T.S., Knudsen, H., Lauritzen, A.F., Nielsen, S.L., Klausen, T.W. & Norgaard, P. (2014) MYC translocation
partner gene determines survival of patients with large B-cell lymphoma with MYC- or double-hit MYC/BCL2 translocations. European Journal of
Haematology, 92, 42–48.
Perry, A.M., Crockett, D., Dave, B.J., Althof, P., Winkler, L., Smith, L.M., Aoun, P., Chan, W.C., Fu, K., Greiner, T.C., Bierman, P., Gregory Bo- ciek, R., Vose, J.M., Armitage, J.O. & Weisenburger, D.D. (2013) B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and burkitt lymphoma: study of 39 cases. British Journal of Haematology, 162, 40–49.
Perry, A.M., Alvarado-Bernal, Y., Laurini, J.A., Smith, L.M., Slack, G.W., Tan, K.L., Sehn, L.H., Fu, K., Aoun, P., Greiner, T.C., Chan, W.C., Bi-erman, P.J., Bociek, R.G., Armitage, J.O., Vose, J.M., Gascoyne, R.D. & Weisenburger, D.D. (2014) MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. British Joural of Haematology, 165, 382–391.
Petrich, A.M., Gandhi, M., Jovanovic, B., Castillo, J.J., Rajguru, S., Yang, D.T., Shah, K.A., Whyman, J.D., Lansigan, F., Hernandez-Ilizaliturri, F.J., Lee, L.X., Barta, S.K., Melinamani, S., Karmali, R., Adeimy, C., Smith, S., Dalal, N., Nabhan, C., Peace, D., Vose, J., Evens, A.M., Shah, N., Fenske, T.S., Zelenetz, A.D., Landsburg, D.J., Howlett, C., Mato, A., Jaglal, M., Chavez, J.C., Tsai, J.P., Reddy, N., Li, S., Handler, C., Flowers, C.R., Cohen, J.B., Blum, K.A., Song, K., Sun, H.L., Press, O., Cassaday, R., Jaso, J., Medeiros, L.J., Sohani, A.R. & Abramson, J.S. (2014) Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood, 124, 2354–2361.
Puvvadda, S.D., Stiff, P.J., LeBlanc, M., Cook, J.R., Kahl, B.S., Li, H., Rimsza, L., Friedberg, J. & Smith, S. (2014) MYC Associated and Double Protein Lymphoma: Subset Analysis of SWOG S9704. In: ASH Annual Meeting Abstracts. Inpress.
Savage, K.J., Johnson, N.A., Ben-Neriah, S., Connors, J.M., Sehn, L.H., Farinha, P., Horsman, D.E. & Gascoyne, R.D. (2009) MYC gene rear- rangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood, 114, 3533–3537.
Savage, K.J., Sehn, L.H., Villa, D., Kansara, R.R., Mottok, A., Ennishi, D., Ben-Neriah, S., Kridel, R., Steidl, C., Tan, K.L., Johnson, N.A., Slack, G.W., Connors, J.M., Farinha, P., Scott, D.W. & Gascoyne, R. (2014) The Impact of Concurrent MYC BCL2 Protein Expression on the Risk of Secondary Central Nervous System Relapse in Diffuse Large B-Cell Lymphoma (DLBCL) In: ASH Annual Meeting Abstracts. In press. Sewastianik, T., Prochorec-Sobieszek, M., Chapuy, B. & Juszczynski, P. (2014) MYC deregulation in lymphoid tumors: molecular mechanisms, clinical consequences and therapeutic implications. Biochimica et Biophysica Acta, 1846, 457467.
Snuderl, M., Kolman, O.K., Chen, Y.B., Hsu, J.J., Ackerman, A.M., Dal Cin, P., Ferry, J.A., Harris, N.L., Hasserjian, R.P., Zukerberg, L.R., Abram-
son, J.S., Hochberg, E.P., Lee, H., Lee, A.I., Too- mey, C.E. & Sohani, A.R. (2010) B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. American Journal of Surgical Pathology, 34, 327–340.
Swerdlow, S.H., Campo, E., Lee Harris, N., Jaffe, E.S., PIleri, S.A., Stein, H., Thiele, J. & Vardiman, J.W. (2008) WHO Classification of Tumors of the Hematopoeitic and Lymphoid Tissues. IARC, Lyon. Tapia, G., Lopez, R., Munoz-Marmol, A.M., Mate, J.L., Sanz, C., Marginet, R., Navarro, J.T., Ribera, J.M. & Ariza, A. (2011) Immunohistochemical detection of MYC protein correlates with MYC gene status in aggressive B cell lymphomas. His- topathology, 59, 672–678.
Thangavelu, M., Olopade, O., Beckman, E., Vardiman, J.W., Larson, R.A., McKeithan, T.W., Le Beau, M.M. & Rowley, J.D. (1990) Clinical, morphologic, and cytogenetic characteristics of patients with lymphoid malignancies characterized by both t(14;18)(q32;q21) and t(8;14)(q24;
q32) or t(8;22)(q24;q11). Genes Chromosomes Cancer, 2, 147–158.
Tibiletti, M.G., Martin, V., Bernasconi, B., Del Curto, B., Pecciarini, L., Uccella, S., Pruneri, G., Ponzoni, M., Mazzucchelli, L., Martinelli, G., Ferreri, A.J., Pinotti, G., Assanelli, A., Scandurra, M., Doglioni, C., Zucca, E., Capella, C. & Bertoni, F. (2009) BCL2, BCL6, MYC, MALT 1, and BCL10 rearrangements in nodal diffuse large B-cell lymphomas: a multicenter evaluation of a new set of fluorescent in situ hybridization probes and correlation with clinical outcome.
Human Pathology, 40, 645–652. Tomita, N., Tokunaka, M., Nakamura, N., Takeuchi, K., Koike, J., Motomura, S., Miyamoto, K., Kikuchi, A., Hyo, R., Yakushijin, Y., Masaki, Y., Fujii, S., Hayashi, T., Ishigatsubo, Y. & Miura, I. (2009) Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 794 ª 2014 John Wiley & Sons Ltd British Journal of Haematology, 2015, 168, 784–795 and MYC translocations. Haematologica, 94,935–943.
Tzankov, A., Xu-Monette, Z.Y., Gerhard, M., Visco, C., Dirnhofer, S., Gisin, N., Dybkaer, K., Orazi, A., Bhagat, G., Richards, K.L., Hsi, E.D., Choi, W.W., van Krieken, J.H., Ponzoni, M., Ferreri, A.J., Ye, Q., Winter, J.N., Farnen, J.P., Piris, M.A., Moller, M.B., You, M.J., McDonnell, T., Medeiros, L.J. & Young, K.H. (2014) Rearrangements of MYC gene facilitate risk stratification in diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Modern Pathology, 27, 958–971.
Valera, A., Lopez-Guillermo, A., Cardesa-Salzmann, T., Climent, F., Gonzalez-Barca, E., Mercadal, S., Espinosa, I., Novelli, S., Briones, J., Mate, J.L., Salamero, O., Sancho, J.M., Arenillas, L., Serrano, S., Erill, N., Martinez, D., Castillo, P., Rovira, J., Martinez, A., Campo, E. & Colomo, L. (2013) MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica, 98, 1554–1562.
Visco, C., Tzankov, A., Xu-Monette, Z.Y., Miranda, R.N., Tai, Y.C., Li, Y., Liu, W.M., d’Amore, E.S., Li, Y., Montes-Moreno, S., Dybkaer, K., Chiu, A., Orazi, A., Zu, Y., Bhagat, G., Wang, H.Y., Dunphy, C.H., His, E.D., Zhao, X.F., Choi, W.W., Zhao, X., van Krieken, J.H., Huang, Q., Ai, W., O’Neill, S., Ponzoni, M., Ferreri, A.J., Kahl, B.S., Winter, J.N., Go, R.S., Dirnhofer, S., Piris, M.A., Moller, M.B., Wu, L., Medeiros, L.J. & Young, K.H. (2013) Patients with diffuse large B-cell lymphoma of germinal center origin with BCL2 translocations have poor outcome, irrespective of MYC status: a report from an International DLBCL rituximab-CHOP Consortium Program Study. Haematologica, 98, 255–263.
Vitolo, U., Chiappella, A., Franceschetti, S., Carella, A.M., Baldi, I., Inghirami, G., Spina, M., Pavone, V., Ladetto, M., Liberati, A.M., Molinari, A.L., Zinzani, P., Salvi, F., Fattori, P.P., Zaccaria, A., Dreyling, M., Botto, B., Castellino, A., Congiu, A., Gaudiano, M., Zanni, M., Ciccone, G., Gaidano, G. & Rossi, G. (2014) Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: results of the REAL07 open-label, multicentre, phase 2 trial. The Lancet Oncology, 15, 730–737.
Vose, J.M., Habermann, T.M., Czuczman, M.S., Zinzani, P.L., Reeder, C.B., Tuscano, J.M., Lossos, I.S., Li, J., Pietronigro, D. & Witzig, T.E. (2013) Single-agent lenalidomide is active in patients with relapsed or refractory aggressive non-Hodgkin lymphoma who received prior stem cell transplantation. British Journal of Haematology, 162, 639–647.
Walker, C.J., Oaks, J.J., Santhanam, R., Neviani, P., Harb, J.G., Ferenchak, G., Ellis, J.J., Landesman, Y., Eisfeld, A.K., Gabrail, N.Y., Smith, C.L., Caligiuri, M.A., Hokland, P., Roy, D.C., Reid, A., Milojkovic, D., Goldman, J.M., Apperley, J., Garzon, R., Marcucci, G., Shacham, S., Kauffman, M.G. & Perrotti, D. (2013) Preclinical and clinical efficacy of XPO1/CRM1 inhibition by the karyopherin inhibitor KPT-330 in Ph+ leukemias. Blood, 122, 3034–3044.
Wang, M., Fowler, N., Wagner-Bartak, N., Feng, L., Romaguera, J., Neelapu, S.S., Hagemeister, F., Fanale, M., Oki, Y., Pro, B., Shah, J., Tho- mas, S., Younes, A., Hosing, C., Zhang, L., Newberry, K.J., Desai, M., Cheng, N., Badillo, M., Bejarano, M., Chen, Y., Young, K.H., Champlin, R., Kwak, L. & Fayad, L. (2013) Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia, 27,1902–1909.
Witzig, T.E., Vose, J.M., Zinzani, P.L., Reeder, C.B., Buckstein, R., Polikoff, J.A., Bouabdallah, R., Haioun, C., Tilly, H., Guo, P., Pietronigro, D., Ervin-Haynes, A.L. & Czuczman, M.S. (2011) An international phase II trial of singleagent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Annals of Oncology, 22, 1622–1627.
Yan, L.X., Liu, Y.H., Luo, D.L., Zhang, F., Cheng, Y., Luo, X.L., Xu, J., Cheng, J. & Zhuang, H.G. (2014) MYC expression in concert with BCL2 and BCL6 expression predicts outcome in Chinese patients with diffuse large B-cell lymphoma, not otherwise specified. PLoS One, 9, e104068. Younes, A., Thieblemont, C., Morschhauser, F., Flinn, I., Friedberg, J.W., Amorim, S., Hivert, B., Westin, J., Vermeulen, J., Bandyopadhyay, N., de Vries, R., Balasubramanian, S., Hellemans, P., Smit, J.W., Fourneau, N. & Oki, Y.(2014) Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatmentnaive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. The Lancet Oncology, 15, 1019–1026.
Yunis, J.J., Oken, M.M., Theologides, A., Howe, R.B. & Kaplan, M.E. (1984) Recurrent chromosomaldefects are found in most patients with non-Hodgkin’s-lymphoma. Cancer Genetics and Cytogenetics, 13, 17–28.
Zhong, Y., El-Gamal, D., Dubovsky, J.A., Beckwith, K.A., Harrington, B.K., Williams, K.E., Goettl, V.M., Jha, S., Mo, X., Jones, J.A., Flynn, J.M., Maddocks, K.J., Andritsos, L.A., McCauley, D., Shacham, S., Kauffman, M., Byrd, J.C. & Lapalombella, R. (2014) Selinexor suppresses down-stream effectors of B-cell activation, proliferation and migration in chronic lymphocytic leukemia cells. Leukemia, 28, 1158–1163.
Zhou, M., Wang, J., Ouyang, J., Xu, J.Y., Chen, B., Zhang, Q.G., Zhou, R.F., Yang, Y.G., Shao, X.Y., Xu, Y., Chen, Y.M., Fan, X.S. & Wu, H.Y. (2014) MYC protein expression is associated with poor prognosis in diffuse large B cell lymphoma patients treated with RCHOP chemotherapy. Tumour Biology, 35, 6757–6762.
Zinzani, P.L., Pellegrini, C., Gandolfi, L., Stefoni, V., Quirini, F., Derenzini, E., Broccoli, A., Argnani, L., Pileri, S. & Baccarani, M. (2011) Com-bination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 2 trial.
Clinical Lymphoma, Myeloma & Leukemia, 11,462–466.





